Possible Roles of Proinflammatory Signaling in Keratinocytes Through Aryl Hydrocarbon Receptor Ligands for the Development of Squamous Cell Carcinoma
- PMID: 33178182
- PMCID: PMC7596320
- DOI: 10.3389/fimmu.2020.534323
Possible Roles of Proinflammatory Signaling in Keratinocytes Through Aryl Hydrocarbon Receptor Ligands for the Development of Squamous Cell Carcinoma
Abstract
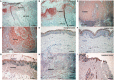
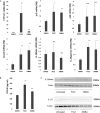
Aryl hydrocarbon receptor (AhR) provides a deeper insight into the pathogenesis of cutaneous squamous cell carcinoma (cSCC). AhR ligands, such as 6-formylindolo[3,2-b] carbazole (FICZ), and 7,12-Dimethylbenz[a]anthracene (DMBA), constitute major substrates for the cytochrome P450 (CYP) family, and influence the expression of various cytokine genes, including IL-17 and IL-23-related genes via the AhR. On the other hand, proinflammatory cytokines could drive tumor progression through the TRAF-ERK5 signaling pathway in cSCC. From the above findings, we hypothesized that AhR ligands might enhance the mRNA expression of proinflammatory cytokines via the AhR, leading to the development of cSCC. The purpose of this study was to investigate (1) the immunomodulatory effects of FICZ and DMBA on normal human keratinocytes (NHKCs), focusing on IL-17, and related cytokines/chemokines (IL-23, IL-36γ, and CCL20), (2) the expression of these factors in AhR-dependent pathways using a two-stage chemically induced skin carcinogenesis mouse model, and (3) the expression of these factors in lesion-affected skin in cSCC. Both FICZ and DMBA augmented the expression of CYP1A1, p19, CCL20, and IL-36γ mRNA in NHKCs in vitro. Moreover, the mRNA expression of these proinflammatory factors, as well as IL-17, in mouse cSCC is significantly decreased in the AhR-(fl/fl) Krt5-(Cre) mice compared to wild type mice, leading to a decrease in the number of developed cSCC lesions. Furthermore, CCL20, IL-23, as well as IL-17, are detected in the lesion-affected skin of cSCC patients. Our study demonstrates a possible mechanism for the development of cSCC involving AhR-mediated signaling by epidermal keratinocytes and recruitment of Th17 cells.
Keywords: IL-17; aryl hydrocarbon receptor; carcinogenesis; cutaneous SCC; proinflammatory cytokines.
Copyright © 2020 Sato, Fujimura, Hidaka, Lyu, Tanita, Matsushita, Yamamoto and Aiba.
Figures


Similar articles
-
Malassezia-derived aryl hydrocarbon receptor ligands enhance the CCL20/Th17/soluble CD163 pathogenic axis in extra-mammary Paget's disease.Exp Dermatol. 2019 Aug;28(8):933-939. doi: 10.1111/exd.13944. Epub 2019 Jun 6. Exp Dermatol. 2019. PMID: 31001887
-
Reduction of CC-chemokine ligand 5 by aryl hydrocarbon receptor ligands.J Dermatol Sci. 2013 Oct;72(1):9-15. doi: 10.1016/j.jdermsci.2013.04.031. Epub 2013 Jun 13. J Dermatol Sci. 2013. PMID: 23810773
-
Editor's Highlight: Ah Receptor Activation Potentiates Neutrophil Chemoattractant (C-X-C Motif) Ligand 5 Expression in Keratinocytes and Skin.Toxicol Sci. 2017 Nov 1;160(1):83-94. doi: 10.1093/toxsci/kfx160. Toxicol Sci. 2017. PMID: 28973351 Free PMC article.
-
The tryptophan derivative 6-formylindolo[3,2-b]carbazole, FICZ, a dynamic mediator of endogenous aryl hydrocarbon receptor signaling, balances cell growth and differentiation.Crit Rev Toxicol. 2018 Aug;48(7):555-574. doi: 10.1080/10408444.2018.1493086. Epub 2018 Sep 18. Crit Rev Toxicol. 2018. PMID: 30226107 Review.
-
Influence of light on aryl hydrocarbon receptor signaling and consequences in drug metabolism, physiology and disease.Expert Opin Drug Metab Toxicol. 2011 Oct;7(10):1267-93. doi: 10.1517/17425255.2011.614947. Epub 2011 Sep 2. Expert Opin Drug Metab Toxicol. 2011. PMID: 21883026 Review.
Cited by
-
Overexpression of CCL-20 and CXCL-8 genes enhances tumor escape and resistance to cemiplimab, a programmed cell death protein-1 (PD-1) inhibitor, in patients with locally advanced and metastatic cutaneous squamous cell carcinoma.Oncoimmunology. 2024 Aug 26;13(1):2388315. doi: 10.1080/2162402X.2024.2388315. eCollection 2024. Oncoimmunology. 2024. PMID: 39206096 Free PMC article.
-
Stromal Factors as a Target for Immunotherapy in Melanoma and Non-Melanoma Skin Cancers.Int J Mol Sci. 2022 Apr 6;23(7):4044. doi: 10.3390/ijms23074044. Int J Mol Sci. 2022. PMID: 35409404 Free PMC article. Review.
-
Aryl Hydrocarbon Receptor as an Anticancer Target: An Overview of Ten Years Odyssey.Molecules. 2023 May 9;28(10):3978. doi: 10.3390/molecules28103978. Molecules. 2023. PMID: 37241719 Free PMC article. Review.
-
Interleukin-38 promotes skin tumorigenesis in an IL-1Rrp2-dependent manner.EMBO Rep. 2022 Jun 7;23(6):e53791. doi: 10.15252/embr.202153791. Epub 2022 May 17. EMBO Rep. 2022. PMID: 35578812 Free PMC article.
-
Distinguishing Keratoacanthoma from Well-Differentiated Cutaneous Squamous Cell Carcinoma Using Single-Cell Spatial Pathology.J Invest Dermatol. 2023 Dec;143(12):2397-2407.e8. doi: 10.1016/j.jid.2023.06.192. Epub 2023 Jul 5. J Invest Dermatol. 2023. PMID: 37419445 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

