Umbilical Cord Blood and iPSC-Derived Natural Killer Cells Demonstrate Key Differences in Cytotoxic Activity and KIR Profiles
- PMID: 33178188
- PMCID: PMC7593774
- DOI: 10.3389/fimmu.2020.561553
Umbilical Cord Blood and iPSC-Derived Natural Killer Cells Demonstrate Key Differences in Cytotoxic Activity and KIR Profiles
Abstract
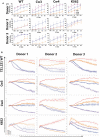
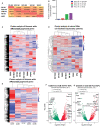
Natural killer (NK) cells derived or isolated from different sources have been gaining in importance for cancer therapies. In this study, we evaluate and compare key characteristics between NK cells derived or isolated from umbilical cord blood, umbilical cord blood hematopoietic stem/progenitor cells, peripheral blood, and induced pluripotent stem cells (iPSCs). Specifically, we find CD56+ NK cells isolated and expanded directly from umbilical cord blood (UCB56) and NK cells derived from CD34+ hematopoietic stem/progenitors in umbilical cord blood (UCB34) differ in their expression of markers associated with differentiation including CD16, CD2, and killer Ig-like receptors (KIRs). UCB56-NK cells also displayed a more potent cytotoxicity compared to UCB34-NK cells. NK cells derived from iPSCs (iPSC-NK cells) were found to have variable KIR expression, with certain iPSC-NK cell populations expressing high levels of KIRs and others not expressing KIRs. Notably, KIR expression on UCB56 and iPSC-NK cells had limited effect on cytotoxic activity when stimulated by tumor target cells that express high levels of cognate HLA class I, suggesting that in vitro differentiation and expansion may override the KIR-HLA class I mediated inhibition when used across HLA barriers. Together our results give a better understanding of the cell surface receptor, transcriptional, and functional differences between NK cells present in umbilical cord blood and hematopoietic progenitor-derived NK cells which may prove important in selecting the most active NK cell populations for treatment of cancer or other therapies.
Keywords: KIRs; cancer; natural killer cells; stem cells; umbilical cord blood.
Copyright © 2020 Goldenson, Zhu, Wang, Heragu, Bernareggi, Ruiz-Cisneros, Bahena, Ask, Hoel, Malmberg and Kaufman.
Figures



Similar articles
-
NK cell killer Ig-like receptor repertoire acquisition and maturation are strongly modulated by HLA class I molecules.J Immunol. 2014 Mar 15;192(6):2602-10. doi: 10.4049/jimmunol.1302843. Epub 2014 Feb 19. J Immunol. 2014. PMID: 24554773
-
NK cell development in a human stem cell niche: KIR expression occurs independently of the presence of HLA class I ligands.Blood Adv. 2018 Oct 9;2(19):2452-2461. doi: 10.1182/bloodadvances.2018019059. Blood Adv. 2018. PMID: 30266820 Free PMC article.
-
IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential.PLoS One. 2014 Jan 31;9(1):e87131. doi: 10.1371/journal.pone.0087131. eCollection 2014. PLoS One. 2014. PMID: 24498025 Free PMC article.
-
Strategies to activate NK cells to prevent relapse and induce remission following hematopoietic stem cell transplantation.Blood. 2018 Mar 8;131(10):1053-1062. doi: 10.1182/blood-2017-08-752170. Epub 2018 Jan 22. Blood. 2018. PMID: 29358179 Free PMC article. Review.
-
KIR specificity and avidity of standard and unusual C1, C2, Bw4, Bw6 and A3/11 amino acid motifs at entire HLA:KIR interface between NK and target cells, the functional and evolutionary classification of HLA class I molecules.Int J Immunogenet. 2019 Aug;46(4):217-231. doi: 10.1111/iji.12433. Epub 2019 Jun 18. Int J Immunogenet. 2019. PMID: 31210416 Review.
Cited by
-
An Alternative Cell Therapy for Cancers: Induced Pluripotent Stem Cell (iPSC)-Derived Natural Killer Cells.Biomedicines. 2021 Sep 26;9(10):1323. doi: 10.3390/biomedicines9101323. Biomedicines. 2021. PMID: 34680440 Free PMC article. Review.
-
Targeted integration of EpCAM-specific CAR in human induced pluripotent stem cells and their differentiation into NK cells.Stem Cell Res Ther. 2021 Nov 21;12(1):580. doi: 10.1186/s13287-021-02648-4. Stem Cell Res Ther. 2021. PMID: 34802459 Free PMC article.
-
Advances in NK cell production.Cell Mol Immunol. 2022 Apr;19(4):460-481. doi: 10.1038/s41423-021-00808-3. Epub 2022 Jan 5. Cell Mol Immunol. 2022. PMID: 34983953 Free PMC article. Review.
-
Engineered iPSC-derived natural killer cells: recent innovations in translational innate anti-cancer immunotherapy.Clin Transl Immunology. 2025 Jul 10;14(7):e70045. doi: 10.1002/cti2.70045. eCollection 2025. Clin Transl Immunology. 2025. PMID: 40655896 Free PMC article. Review.
-
Natural Killer Cell-Mediated Immunotherapy for Leukemia.Cancers (Basel). 2022 Feb 8;14(3):843. doi: 10.3390/cancers14030843. Cancers (Basel). 2022. PMID: 35159109 Free PMC article. Review.
References
-
- Miller JS, Lanier LL. Natural killer cells in cancer immunotherapy. Annu Rev Cancer Biol. (2019) 3:77–103. 10.1146/annurev-cancerbio-030518-055653 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

