Use of T Cell Mediated Immune Functional Assays for Adjustment of Immunosuppressive or Anti-infective Agents in Solid Organ Transplant Recipients: A Systematic Review
- PMID: 33178194
- PMCID: PMC7593245
- DOI: 10.3389/fimmu.2020.567715
Use of T Cell Mediated Immune Functional Assays for Adjustment of Immunosuppressive or Anti-infective Agents in Solid Organ Transplant Recipients: A Systematic Review
Abstract
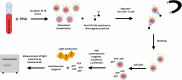
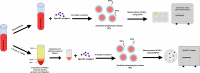
Background: Defining the optimal dosage of the immunosuppressive or duration of anti-infective agents is a challenge in solid organ transplant (SOT) recipients. We aimed to systematically review the literature regarding the use of T cell mediated immune functional assays (IFAs) for adjustment of the immunosuppressive or anti-infective agents in SOT recipients. Methods: We systematically searched PubMed, Scopus, EMBASE, Web of Science (WOS), Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov to find human interventional studies or study protocols that used either in-house or commercially available IFAs for adjustment of the immunosuppressive or anti-infective agents in SOT recipients. Results: We included six clinical trials and six study protocols. Four out of the six clinical trials used interferon-γ release assays for cytomegalovirus (IGRA-CMV), and five out of the six registered study protocols planned to use IGRA-CMV for adjustment of anti-CMV antiviral (Valganciclovir) prophylaxis or preemptive therapy in SOT recipients. Primary or secondary anti-CMV prophylaxes were discontinued in SOT recipients who had positive IGRA-CMV results without an increase in the rate of CMV infection or reactivation. Among other IFAs, one clinical trial used interferon-γ release assays for tuberculosis (IGRA-TB), and one study used ImmuKnow for adjustment of the duration and dosage of isoniazid and tacrolimus, respectively. Conclusion: Our systematic review supports a promising role for the IGRA-CMVs for adjustment of the duration of anti-CMV antiviral prophylaxis in SOT recipients. There are limited data to support the use of IFAs other than IGRA-CMVs for adjustment of immunosuppressive or anti-infective agents. Further multicenter randomized clinical trials using IFAs other than IGRA-CMVs may help in personalized immunosuppressive or prophylactic anti-infective therapy in SOT recipients.
Keywords: anti-infective agent; immune functional assay; immune system; immunosuppressive agent; transplantation.
Copyright © 2020 Rezahosseini, Møller, Knudsen, Sørensen, Perch, Gustafsson, Rasmussen, Ostrowski and Nielsen.
Figures


Similar articles
-
Viral load, CMV-specific T-cell immune response and cytomegalovirus disease in solid organ transplant recipients at higher risk for cytomegalovirus infection during preemptive therapy.Transpl Int. 2014 Oct;27(10):1060-8. doi: 10.1111/tri.12378. Epub 2014 Aug 20. Transpl Int. 2014. PMID: 24964364
-
Cytomegalovirus Infection After Solid Organ Transplantation: How I Use Cell-Mediated Immune Assays for Management.Viruses. 2024 Nov 15;16(11):1781. doi: 10.3390/v16111781. Viruses. 2024. PMID: 39599895 Free PMC article. Review.
-
Prevention of cytomegalovirus following solid organ transplantation: a literature review.Pediatr Transplant. 2013 Sep;17(6):499-509. doi: 10.1111/petr.12118. Pediatr Transplant. 2013. PMID: 23890075 Review.
-
[Monitoring of cytomegalovirus-specific CD4+ and CD8+ T cell responses by cytokine flow cytometry in renal transplant recipients].Mikrobiyol Bul. 2016 Apr;50(2):224-35. Mikrobiyol Bul. 2016. PMID: 27175495 Turkish.
-
Effect of long-term prophylaxis in the development of cytomegalovirus-specific T-cell immunity in D+/R- solid organ transplant recipients.Transpl Infect Dis. 2015 Oct;17(5):637-46. doi: 10.1111/tid.12417. Epub 2015 Aug 27. Transpl Infect Dis. 2015. PMID: 26134282
Cited by
-
Torque teno virus as a marker of immune status in immunocompromised patients: A systematic review.Eur J Clin Invest. 2025 Aug;55(8):e70068. doi: 10.1111/eci.70068. Epub 2025 May 15. Eur J Clin Invest. 2025. PMID: 40371633 Free PMC article.
-
Laboratory diagnostic testing for cytomegalovirus infection in solid organ transplant patients.Korean J Transplant. 2022 Mar 31;36(1):15-28. doi: 10.4285/kjt.22.0001. Korean J Transplant. 2022. PMID: 35769434 Free PMC article. Review.
-
Regulation of the immune microenvironment and immunotherapy after liver transplantation.Front Immunol. 2025 May 12;16:1602877. doi: 10.3389/fimmu.2025.1602877. eCollection 2025. Front Immunol. 2025. PMID: 40421010 Free PMC article. Review.
-
Case Report: Cytomegalovirus Disease Is an Under-Recognized Contributor to Morbidity and Mortality in Common Variable Immunodeficiency.Front Immunol. 2022 Feb 15;13:815193. doi: 10.3389/fimmu.2022.815193. eCollection 2022. Front Immunol. 2022. PMID: 35242131 Free PMC article.
-
Prediction of herpes virus infections after solid organ transplantation: a prospective study of immune function.Front Immunol. 2023 Jul 3;14:1183703. doi: 10.3389/fimmu.2023.1183703. eCollection 2023. Front Immunol. 2023. PMID: 37465673 Free PMC article.
References
-
- Drabe CH, Sorensen SS, Rasmussen A, Perch M, Gustafsson F, Rezahosseini O, et al. . Immune function as predictor of infectious complications and clinical outcome in patients undergoing solid organ transplantation (the ImmuneMo:SOT study): a prospective non-interventional observational trial. BMC Infect Dis. (2019) 19:573. 10.1186/s12879-019-4207-9 - DOI - PMC - PubMed
-
- Enderby C, Keller CA. An overview of immunosuppression in solid organ transplantation. Am J Manag Care. (2015) 21:s12–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

