Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the cGAS-STING Pathway
- PMID: 33178195
- PMCID: PMC7593769
- DOI: 10.3389/fimmu.2020.571334
Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the cGAS-STING Pathway
Abstract
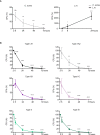
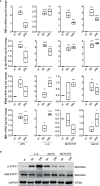
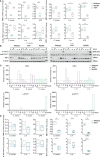
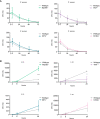
Cutibacterium (previously Propionibacterium) acnes is an anaerobic, Gram-positive commensal of the human body. The bacterium has been associated with a variety of diseases, including acne vulgaris, prosthetic joint infections, prostate cancer, and sarcoidosis. The accumulation of C. acnes in diseases such as acne and prostate cancer has been shown to correlate with enhanced inflammation. While the C. acnes-induced proinflammatory axis, via NF-κB and MAPK signaling and inflammasome activation, has been investigated over the last few decades, the potential role of C. acnes in triggering the type I interferon (IFN-I) pathway has not been addressed. Our results show that C. acnes induces the IFN-I signaling axis in human macrophages by triggering the cGAS-STING pathway. In addition, IFN-I signaling induced by C. acnes strongly depends on the adapter protein TRIF in a non-canonical manner; these signaling events occurred in the absence of any detectable intracellular replication of the bacterium. Collectively, our results provide important insight into C. acnes-induced intracellular signaling cascades in human macrophages and suggest IFN-I as a factor in the etiology of C. acnes-induced diseases. This knowledge may be valuable for developing novel therapies targeting C. acnes in diseases where the accumulation of the bacterium leads to an inflammatory pathology.
Keywords: Cutibacterium acnes; Listeria (L.) monocytogenes; NFkB = nuclear factor kappa b; STAT (signal transducer and activator of transcription); TIR-domain-containing adapter-inducing interferon-β; cyclic GMP-AMP synthase; interferon; stimulator of interferon genes.
Copyright © 2020 Fischer, Tschismarov, Pilz, Straubinger, Carotta, McDowell and Decker.
Figures




Similar articles
-
Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article.
-
Brucella abortus Triggers a cGAS-Independent STING Pathway To Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation.J Immunol. 2018 Jan 15;200(2):607-622. doi: 10.4049/jimmunol.1700725. Epub 2017 Dec 4. J Immunol. 2018. PMID: 29203515 Free PMC article.
-
cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection.J Immunol. 2015 Apr 1;194(7):3236-45. doi: 10.4049/jimmunol.1402764. Epub 2015 Feb 20. J Immunol. 2015. PMID: 25710914 Free PMC article.
-
COVID-19 Molecular Pathophysiology: Acetylation of Repurposing Drugs.Int J Mol Sci. 2022 Oct 31;23(21):13260. doi: 10.3390/ijms232113260. Int J Mol Sci. 2022. PMID: 36362045 Free PMC article. Review.
-
The regulation of cGAS-STING signaling by RNA virus-derived components.Virol J. 2024 May 1;21(1):101. doi: 10.1186/s12985-024-02359-1. Virol J. 2024. PMID: 38693578 Free PMC article. Review.
Cited by
-
Alterations in the nasopharyngeal microbiome associated with SARS-CoV-2 infection status and disease severity.PLoS One. 2022 Oct 14;17(10):e0275815. doi: 10.1371/journal.pone.0275815. eCollection 2022. PLoS One. 2022. PMID: 36240246 Free PMC article.
-
The Role of cGAS-STING in Age-Related Diseases from Mechanisms to Therapies.Aging Dis. 2023 Aug 1;14(4):1145-1165. doi: 10.14336/AD.2023.0117. Aging Dis. 2023. PMID: 37163421 Free PMC article. Review.
-
NeoGenesis MB-1 with CRISPR Technology Reduces the Effects of the Viruses (Phages) Associated with Acne - Case Report.Integr Med (Encinitas). 2024 Sep;23(4):34-38. Integr Med (Encinitas). 2024. PMID: 39355416
-
Macrophages in acne vulgaris: mediating phagocytosis, inflammation, scar formation, and therapeutic implications.Front Immunol. 2024 Mar 14;15:1355455. doi: 10.3389/fimmu.2024.1355455. eCollection 2024. Front Immunol. 2024. PMID: 38550588 Free PMC article. Review.
-
Prostate and urinary microbiomes in prostate cancer development: focus on Cutibacterium acnes.Front Cell Infect Microbiol. 2025 May 21;15:1562729. doi: 10.3389/fcimb.2025.1562729. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40470262 Free PMC article. Review.
References
-
- McDowell A, Nagy I. Molecular Medical Microbiology. Part 7 Localized Infect (2015) 1:837–58. 10.1016/b978-0-12-397169-2.00046-9 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

