Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-κB Pathway
- PMID: 33178197
- PMCID: PMC7592646
- DOI: 10.3389/fimmu.2020.571959
Threonyl-tRNA Synthetase Promotes T Helper Type 1 Cell Responses by Inducing Dendritic Cell Maturation and IL-12 Production via an NF-κB Pathway
Abstract
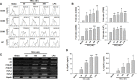
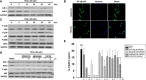
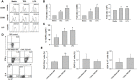
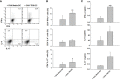
Threonyl-tRNA synthetase (TRS) is an aminoacyl-tRNA synthetase that catalyzes the aminoacylation of tRNA by transferring threonine. In addition to an essential role in translation, TRS was extracellularly detected in autoimmune diseases and also exhibited pro-angiogenetic activity. TRS is reported to be secreted into the extracellular space when vascular endothelial cells encounter tumor necrosis factor-α. As T helper (Th) type 1 response and IFN-γ levels are associated with autoimmunity and angiogenesis, in this study, we investigated the effects of TRS on dendritic cell (DC) activation and CD4 T cell polarization. TRS-treated DCs exhibited up-regulated expression of activation-related cell-surface molecules, including CD40, CD80, CD86, and MHC class II. Treatment of DCs with TRS resulted in a significant increase of IL-12 production. TRS triggered nuclear translocation of the NF-κB p65 subunit along with the degradation of IκB proteins and the phosphorylation of MAPKs in DCs. Additionally, MAPK inhibitors markedly recovered the degradation of IκB proteins and the increased IL-12 production in TRS-treated DCs, suggesting the involvement of MAPKs as the upstream regulators of NF-κB in TRS-induced DC maturation and activation. Importantly, TRS-stimulated DCs significantly increased the populations of IFN-γ+CD4 T cells, and the levels of IFN-γ when co-cultured with CD4+ T cells. The addition of a neutralizing anti-IL-12 mAb to the cell cultures of TRS-treated DCs and CD4+ T cells resulted in decreased IFN-γ production, indicating that TRS-stimulated DCs may enhance the Th1 response through DC-derived IL-12. Injection of OT-II mice with OVA-pulsed, TRS-treated DCs also enhanced Ag-specific Th1 responses in vivo. Importantly, injection with TRS-treated DC exhibited increased populations of IFN-γ+-CD4+ and -CD8+ T cells as well as secretion level of IFN-γ, resulting in viral clearance and increased survival periods in mice infected with influenza A virus (IAV), as the Th1 response is associated with the enhanced cellular immunity, including anti-viral activity. Taken together, these results indicate that TRS promotes the maturation and activation of DCs, DC-mediated Th1 responses, and anti-viral effect on IAV infection.
Keywords: aminoacyl-tRNA synthetase; dendritic cell; influenza A virus; interferon-γ; interleukin-12; threonyl-tRNA synthetase; type 1 helper T cells.
Copyright © 2020 Jung, Park, Cho, Jung, Cho and Kim.
Figures


Similar articles
-
Lysyl-Transfer RNA Synthetase Induces the Maturation of Dendritic Cells through MAPK and NF-κB Pathways, Strongly Contributing to Enhanced Th1 Cell Responses.J Immunol. 2018 Nov 1;201(9):2832-2841. doi: 10.4049/jimmunol.1800386. Epub 2018 Oct 1. J Immunol. 2018. PMID: 30275047
-
AIMP1/p43 protein induces the maturation of bone marrow-derived dendritic cells with T helper type 1-polarizing ability.J Immunol. 2008 Mar 1;180(5):2894-902. doi: 10.4049/jimmunol.180.5.2894. J Immunol. 2008. PMID: 18292511
-
Adiponectin induces dendritic cell activation via PLCγ/JNK/NF-κB pathways, leading to Th1 and Th17 polarization.J Immunol. 2012 Mar 15;188(6):2592-601. doi: 10.4049/jimmunol.1102588. Epub 2012 Feb 15. J Immunol. 2012. PMID: 22345647
-
Interleukin-12 and the regulation of innate resistance and adaptive immunity.Nat Rev Immunol. 2003 Feb;3(2):133-46. doi: 10.1038/nri1001. Nat Rev Immunol. 2003. PMID: 12563297 Review.
-
HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells.Cell Death Differ. 2012 Jan;19(1):96-106. doi: 10.1038/cdd.2011.134. Epub 2011 Oct 28. Cell Death Differ. 2012. PMID: 22033335 Free PMC article. Review.
Cited by
-
Identification of Hub Genes for Dexmedetomidine Alleviation of Limb Ischemia-Reperfusion-Induced Lung Injury in Rats by Transcriptomic.J Inflamm Res. 2025 Apr 24;18:5427-5445. doi: 10.2147/JIR.S512536. eCollection 2025. J Inflamm Res. 2025. PMID: 40297546 Free PMC article.
-
Delivery of antigen to porcine dendritic cells by fusing antigen with porcine dendritic cells targeting peptide.Front Immunol. 2022 Sep 8;13:926279. doi: 10.3389/fimmu.2022.926279. eCollection 2022. Front Immunol. 2022. PMID: 36159835 Free PMC article.
-
Aminoacyl-tRNA synthetase interactions in SARS-CoV-2 infection.Biochem Soc Trans. 2023 Dec 20;51(6):2127-2141. doi: 10.1042/BST20230527. Biochem Soc Trans. 2023. PMID: 38108455 Free PMC article. Review.
-
Aminoacyl-tRNA Synthetase: A Non-Negligible Molecule in RNA Viral Infection.Viruses. 2022 Mar 15;14(3):613. doi: 10.3390/v14030613. Viruses. 2022. PMID: 35337020 Free PMC article. Review.
-
Host Non-Coding RNA Regulates Influenza A Virus Replication.Viruses. 2021 Dec 29;14(1):51. doi: 10.3390/v14010051. Viruses. 2021. PMID: 35062254 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

