Ancient but Not Forgotten: New Insights Into MPEG1, a Macrophage Perforin-Like Immune Effector
- PMID: 33178209
- PMCID: PMC7593815
- DOI: 10.3389/fimmu.2020.581906
Ancient but Not Forgotten: New Insights Into MPEG1, a Macrophage Perforin-Like Immune Effector
Abstract
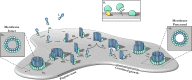
Macrophage-expressed gene 1 [MPEG1/Perforin-2 (PRF2)] is an ancient metazoan protein belonging to the Membrane Attack Complex/Perforin (MACPF) branch of the MACPF/Cholesterol Dependent Cytolysin (CDC) superfamily of pore-forming proteins (PFPs). MACPF/CDC proteins are a large and extremely diverse superfamily that forms large transmembrane aqueous channels in target membranes. In humans, MACPFs have known roles in immunity and development. Like perforin (PRF) and the membrane attack complex (MAC), MPEG1 is also postulated to perform a role in immunity. Indeed, bioinformatic studies suggest that gene duplications of MPEG1 likely gave rise to PRF and MAC components. Studies reveal partial or complete loss of MPEG1 causes an increased susceptibility to microbial infection in both cells and animals. To this end, MPEG1 expression is upregulated in response to proinflammatory signals such as tumor necrosis factor α (TNFα) and lipopolysaccharides (LPS). Furthermore, germline mutations in MPEG1 have been identified in connection with recurrent pulmonary mycobacterial infections in humans. Structural studies on MPEG1 revealed that it can form oligomeric pre-pores and pores. Strikingly, the unusual domain arrangement within the MPEG1 architecture suggests a novel mechanism of pore formation that may have evolved to guard against unwanted lysis of the host cell. Collectively, the available data suggest that MPEG1 likely functions as an intracellular pore-forming immune effector. Herein, we review the current understanding of MPEG1 evolution, regulation, and function. Furthermore, recent structural studies of MPEG1 are discussed, including the proposed mechanisms of action for MPEG1 bactericidal activity. Lastly limitations, outstanding questions, and implications of MPEG1 models are explored in the context of the broader literature and in light of newly available structural data.
Keywords: MACPF domain; MACPF/CDC; MPEG1; PRF2; immune effector; immunology; pore-forming protein.
Copyright © 2020 Bayly-Jones, Pang, Spicer, Whisstock and Dunstone.
Figures
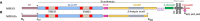




Similar articles
-
Breaching the Bacterial Envelope: The Pivotal Role of Perforin-2 (MPEG1) Within Phagocytes.Front Immunol. 2021 Feb 22;12:597951. doi: 10.3389/fimmu.2021.597951. eCollection 2021. Front Immunol. 2021. PMID: 33692780 Free PMC article. Review.
-
Cholesterol-dependent cytolysins.Adv Exp Med Biol. 2010;677:56-66. doi: 10.1007/978-1-4419-6327-7_5. Adv Exp Med Biol. 2010. PMID: 20687480 Review.
-
Giant Triton Snail Charonia tritonis Macrophage-Expressed Gene 1 Protein Ct-Mpeg1: Molecular Identification, Expression Analysis, and Antimicrobial Activity.Int J Mol Sci. 2022 Nov 2;23(21):13415. doi: 10.3390/ijms232113415. Int J Mol Sci. 2022. PMID: 36362196 Free PMC article.
-
The membrane attack complex, perforin and cholesterol-dependent cytolysin superfamily of pore-forming proteins.J Cell Sci. 2016 Jun 1;129(11):2125-33. doi: 10.1242/jcs.182741. Epub 2016 May 13. J Cell Sci. 2016. PMID: 27179071 Review.
-
Perforin-2/Mpeg1 and other pore-forming proteins throughout evolution.J Leukoc Biol. 2015 Nov;98(5):761-8. doi: 10.1189/jlb.4MR1114-523RR. Epub 2015 Aug 25. J Leukoc Biol. 2015. PMID: 26307549 Free PMC article. Review.
Cited by
-
Combined Inhibition of the TGF-β1/Smad Pathway by Prevotella copri and Lactobacillus murinus to Reduce Inflammation and Fibrosis in Primary Sclerosing Cholangitis.Int J Mol Sci. 2023 Jul 2;24(13):11010. doi: 10.3390/ijms241311010. Int J Mol Sci. 2023. PMID: 37446187 Free PMC article.
-
Olfactory Nerve Transection Transiently Activates Olfactory Ensheathing Cells in Xenopus laevis Larvae.Eur J Neurosci. 2025 Aug;62(3):e70211. doi: 10.1111/ejn.70211. Eur J Neurosci. 2025. PMID: 40758329 Free PMC article.
-
Accumulation and Phagocytosis of Fluorescently Visualized Macrophages Against Edwardsiella piscicida Infection in Established mpeg1.1-Transgenic Japanese Medaka Oryzias latipes.Mar Biotechnol (NY). 2024 Aug;26(4):658-671. doi: 10.1007/s10126-024-10333-9. Epub 2024 Jun 18. Mar Biotechnol (NY). 2024. PMID: 38888725
-
A rare case of Yersinia pseudotuberculosis liver abscess and bacteremia in a heterozygous carrier of HFE1 H63D and MPEG1 mutations in Turkiye.Hepatol Forum. 2024 Oct 25;6(1):22-25. doi: 10.14744/hf.2023.2023.0041. eCollection 2025. Hepatol Forum. 2024. PMID: 40255958 Free PMC article.
-
Transcriptomics of Various Diseases Reveals the Core Role of Immune System Pathways in Retinal Damage Repair and Nerve Regeneration.Mol Neurobiol. 2025 Aug;62(8):10935-10953. doi: 10.1007/s12035-025-04929-y. Epub 2025 Apr 17. Mol Neurobiol. 2025. PMID: 40244560
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

