Spinal Reflex Recovery after Dorsal Rhizotomy and Repair with Platelet-Rich Plasma (PRP) Gel Combined with Bioengineered Human Embryonic Stem Cells (hESCs)
- PMID: 33178285
- PMCID: PMC7647752
- DOI: 10.1155/2020/8834360
Spinal Reflex Recovery after Dorsal Rhizotomy and Repair with Platelet-Rich Plasma (PRP) Gel Combined with Bioengineered Human Embryonic Stem Cells (hESCs)
Abstract
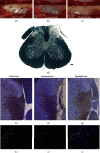
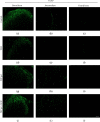
Dorsal root rhizotomy (DRZ) is currently considered an untreatable injury, resulting in the loss of sensitive function and usually leading to neuropathic pain. In this context, we recently proposed a new surgical approach to treat DRZ that uses platelet-rich plasma (PRP) gel to restore the spinal reflex. Success was correlated with the reentry of primary afferents into the spinal cord. Here, aiming to enhance previous results, cell therapy with bioengineered human embryonic stem cells (hESCs) to overexpress fibroblast growth factor 2 (FGF2) was combined with PRP. For these experiments, adult female rats were submitted to a unilateral rhizotomy of the lumbar spinal dorsal roots, which was followed by root repair with PRP gel with or without bioengineered hESCs. One week after DRZ, the spinal cords were processed to evaluate changes in the glial response (GFAP and Iba-1) and excitatory synaptic circuits (VGLUT1) by immunofluorescence. Eight weeks postsurgery, the lumbar intumescences were processed for analysis of the repaired microenvironment by transmission electron microscopy. Spinal reflex recovery was evaluated by the electronic Von Frey method for eight weeks. The transcript levels for human FGF2 were over 37-fold higher in the induced hESCs than in the noninduced and the wildtype counterparts. Altogether, the results indicate that the combination of hESCs with PRP gel promoted substantial and prominent axonal regeneration processes after DRZ. Thus, the repair of dorsal roots, if done appropriately, may be considered an approach to regain sensory-motor function after dorsal root axotomy.
Copyright © 2020 Mateus Vidigal de Castro et al.
Conflict of interest statement
The authors declare that there is no conflict of interests regarding the publication of this paper.
Figures





References
-
- Wu L., Wu J., Chang H. H., Havton L. A. Selective plasticity of primary afferent innervation to the dorsal horn and autonomic nuclei following lumbosacral ventral root avulsion and reimplantation in long term studies. Experimental Neurology. 2012;233(2):758–766. doi: 10.1016/j.expneurol.2011.11.034. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

