Gyejigachulbutang (Gui-Zhi-Jia-Shu-Fu-Tang, Keishikajutsubuto, TJ-18) in Degenerative Knee Osteoarthritis Patients: Lessons and Responders from a Multicenter Randomized Placebo-Controlled Double-Blind Clinical Trial
- PMID: 33178309
- PMCID: PMC7647757
- DOI: 10.1155/2020/2376581
Gyejigachulbutang (Gui-Zhi-Jia-Shu-Fu-Tang, Keishikajutsubuto, TJ-18) in Degenerative Knee Osteoarthritis Patients: Lessons and Responders from a Multicenter Randomized Placebo-Controlled Double-Blind Clinical Trial
Abstract
Background: Gyejigachulbutang (GUI-ZHI-JIA-SHU-FU-TANG, GCB) is an herbal formula widely prescribed in traditional East Asian medicine practice for arthritis and muscle pain. We evaluated the efficacy and safety of GCB for degenerative knee osteoarthritis (KOA).
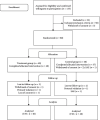
Methods: Eighty patients with KOA were randomly assigned to the GCB group or the placebo group in a 1 : 1 ratio in two Korean medicine hospitals. Patients took GCB or placebo three times a day for 4 weeks. Primary outcome was the change in the visual analogue scale (VAS) score for knee pain from baseline to 4th week. Secondary outcomes were the change in the VAS score from baseline to 2nd week and 8th week, Korean Western Ontario and McMaster Universities Osteoarthritis Index (K-WOMAC), European Quality of Life Five Dimensions questionnaire (EQ-5D), and safety.
Results: There was no significant difference between the compared indicators of the GCB and placebo groups. However, in subgroup analysis, GCB was effective for subjects with a BMI lower than 25 kg/m2. The dose of pain medication was significantly lower in the GCB group than in the placebo group after four weeks (p=0.016). There were no serious adverse events in the GCB group.
Conclusions: GCB was not effective in primary outcome analysis. In exploratory subgroup analysis, GCB might be effective for individuals with BMI lower than 25 kg/m2 for the treatment of degenerative KOA. GCB may also help reduce the consumption of pain medication. Furthermore, research is required for our hypothesis. This trial is registered with KCT0003024.
Copyright © 2020 Myung Kwan Kim et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
References
-
- The Korean Orthpaedic Association. Orthopaedics. 7th. Seoul, Republic of Korea: The New Medical; 2013.
-
- Korean Acupuncture and Moxibustion Medicine Society. The Acupuncture and Moxibustion Medicine. Paju, Republic of Korea: Jipmoondang; 2012.
-
- Bruyère O., Cooper C., Pelletier J.-P., et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Seminars in Arthritis and Rheumatism. 2014;44(3):253–263. doi: 10.1016/j.semarthrit.2014.05.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources