COVID-19 in cancer patients on systemic anti-cancer therapies: outcomes from the CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London) cohort study
- PMID: 33178336
- PMCID: PMC7592172
- DOI: 10.1177/1758835920971147
COVID-19 in cancer patients on systemic anti-cancer therapies: outcomes from the CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London) cohort study
Abstract
Background: Patients with cancer are hypothesised to be at increased risk of contracting COVID-19, leading to changes in treatment pathways in those treated with systemic anti-cancer treatments (SACT). This study investigated the outcomes of patients receiving SACT to assess whether they were at greater risk of contracting COVID-19 or having more severe outcomes.
Methods: Data was collected from all patients receiving SACT in two cancer centres as part of CAPITOL (COVID-19 Cancer PatIenT Outcomes in North London). The primary outcome was the effect of clinical characteristics on the incidence and severity of COVID-19 infection in patients on SACT. We used univariable and multivariable models to analyse outcomes, adjusting for age, gender and comorbidities.
Results: A total of 2871 patients receiving SACT from 2 March to 31 May 2020 were analysed; 68 (2.4%) were diagnosed with COVID-19. Cancer patients receiving SACT were more likely to die if they contracted COVID-19 than those who did not [adjusted (adj.) odds ratio (OR) 9.84; 95% confidence interval (CI) 5.73-16.9]. Receiving chemotherapy increased the risk of developing COVID-19 (adj. OR 2.99; 95% CI = 1.72-5.21), with high dose chemotherapy significantly increasing risk (adj. OR 2.36, 95% CI 1.35-6.48), as did the presence of comorbidities (adj. OR 2.29; 95% CI 1.19-4.38), and having a respiratory or intrathoracic neoplasm (adj. OR 2.12; 95% CI 1.04-4.36). Receiving targeted treatment had a protective effect (adj. OR 0.53; 95% CI 0.30-0.95). Treatment intent (curative versus palliative), hormonal- or immunotherapy and solid versus haematological cancers had no significant effect on risk.
Conclusion: Patients on SACT are more likely to die if they contract COVID-19. Those on chemotherapy, particularly high dose chemotherapy, are more likely to contract COVID-19, while targeted treatment appears to be protective.
Keywords: COVID-19; SACT; SARS-CoV-2; cancer; chemotherapy; coronavirus; hormone therapy; immunotherapy; novel coronavirus; oncology; systemic anti-cancer treatment; targeted treatment; tumour.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: NJH reports grants from CRUK Clinical Trial Fellowship, outside the submitted work. VEC, WW, TAF, SD, JB, KK, DH, KKS, RK, NC, MD, EB, JB, MF and DH have no conflicts of interest to disclose.
Figures




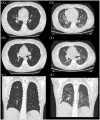
References
-
- World Health Organisation. COVID-19 information [Internet], https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (2020, accessed 22 August 2020).
-
- World Health Organisation. UK COVID-19 information and statistics [Internet], https://covid19.who.int/region/euro/country/gb (2020, accessed 31 August 2020).
-
- Office for National Statistics. Coronavirus (COVID-19) infection survey: characteristics of people testing positive for COVID-19 in England, August 2020. [Internet], https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/... (2020, accessed 22 August 2020).
LinkOut - more resources
Full Text Sources
Miscellaneous

