Donor Lymphocyte Infusions After Allogeneic Stem Cell Transplantation in Acute Leukemia: A Survey From the Gruppo Italiano Trapianto Midollo Osseo (GITMO)
- PMID: 33178602
- PMCID: PMC7593406
- DOI: 10.3389/fonc.2020.572918
Donor Lymphocyte Infusions After Allogeneic Stem Cell Transplantation in Acute Leukemia: A Survey From the Gruppo Italiano Trapianto Midollo Osseo (GITMO)
Abstract
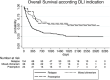
We conducted a retrospective multicenter study including pediatric and adult patients with acute leukemia (AL) who received donor lymphocyte infusions (DLIs) after allogeneic hematopoietic stem cell transplantation (HCT) between January 1, 2010 and December 31, 2015, in order to determine the efficacy and toxicity of the immune treatment. Two hundred fifty-two patients, median age 45.1 years (1.6-73.4), were enrolled from 34 Italian transplant centers. The underlying disease was acute myeloid leukemia in 180 cases (71%). Donors were HLA identical or 1 locus mismatched sibling (40%), unrelated (40%), or haploidentical (20%). The first DLI was administered at a median time of 258 days (55-3,784) after HCT. The main indication for DLI was leukemia relapse (73%), followed by mixed chimerism (17%), and pre-emptive/prophylactic use (10%). Ninety-six patients (38%) received one single infusion, whereas 65 (26%), 42 (17%), and 49 patients (19%) received 2, 3, or ≥4 infusions, respectively, with a median of 31 days between two subsequent DLIs. Forty percent of evaluable patients received no treatment before the first DLI, whereas radiotherapy, conventional chemotherapy or targeted treatments were administered in 3, 39, and 18%, respectively. In informative patients, a few severe adverse events were reported: grade III-IV graft versus host disease (GVHD) (3%), grade III-IV hematological toxicity (11%), and DLI-related mortality (9%). Forty-six patients (18%) received a second HCT after a median of 232 days (32-1,390) from the first DLI. With a median follow-up of 461 days (2-3,255) after the first DLI, 1-, 3-, and 5- year overall survival (OS) of the whole group from start of DLI treatment was 55, 39, and 33%, respectively. In multivariate analysis, older recipient age, and transplants from haploidentical donors significantly reduced OS, whereas DLI for mixed chimerism or as pre-emptive/prophylactic treatment compared to DLI for AL relapse and a schedule including more than one DLI significantly prolonged OS. This GITMO survey confirms that DLI administration in absence of overt hematological relapse and multiple infusions are associated with a favorable outcome in AL patients. DLI from haploidentical donors had a poor outcome and may represent an area of further investigation.
Keywords: acute leukemia; allogeneic stem cell transplantation; donor lymphocyte infusions; pre-emptive treatment; relapse.
Copyright © 2020 Patriarca, Sperotto, Lorentino, Oldani, Mammoliti, Isola, Picardi, Arcese, Saporiti, Sorasio, Mordini, Cavattoni, Musso, Borghero, Micò, Fanin, Bruno, Ciceri and Bonifazi.
Figures
Similar articles
-
Novel Drug Combinations and Donor Lymphocyte Infusions Allow Prolonged Disease Control in Multiple Myeloma Patients Relapsing after Allogeneic Transplantation.Transplant Cell Ther. 2025 Jan;31(1):26.e1-26.e13. doi: 10.1016/j.jtct.2024.10.015. Epub 2024 Nov 4. Transplant Cell Ther. 2025. PMID: 39505212
-
Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-versus-Host Disease-Guided Multiple DLIs Could Improve Outcomes after Allogeneic Hematopoietic Stem Cell Transplantation in Patients with Refractory/Relapsed Acute Leukemia.Biol Blood Marrow Transplant. 2017 Aug;23(8):1311-1319. doi: 10.1016/j.bbmt.2017.04.028. Epub 2017 May 5. Biol Blood Marrow Transplant. 2017. PMID: 28483716 Clinical Trial.
-
Comparison of the safety and efficacy of prophylactic donor lymphocyte infusion after haploidentical versus matched-sibling PBSCT in very high-risk acute myeloid leukemia.Ann Hematol. 2019 May;98(5):1267-1277. doi: 10.1007/s00277-019-03636-8. Epub 2019 Feb 12. Ann Hematol. 2019. PMID: 30747249 Clinical Trial.
-
Pre-emptive and prophylactic donor lymphocyte infusion following allogeneic stem cell transplantation.Int J Hematol. 2023 Aug;118(2):158-168. doi: 10.1007/s12185-023-03595-x. Epub 2023 Apr 4. Int J Hematol. 2023. PMID: 37014602 Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives.J Clin Med. 2022 Jan 4;11(1):253. doi: 10.3390/jcm11010253. J Clin Med. 2022. PMID: 35011994 Free PMC article. Review.
-
Adoptive Cellular Therapies in Pediatric Leukemia Patients After Allogeneic-Hematopoietic Stem Cell Transplants.Immune Netw. 2025 Aug 12;25(4):e29. doi: 10.4110/in.2025.25.e29. eCollection 2025 Aug. Immune Netw. 2025. PMID: 40917789 Free PMC article. Review.
-
Graft-Versus-Host Disease Mouse Models: A Clinical-Translational Perspective.Methods Mol Biol. 2025;2907:1-56. doi: 10.1007/978-1-0716-4430-0_1. Methods Mol Biol. 2025. PMID: 40100591 Review.
-
Expansion, persistence, and efficacy of donor memory-like NK cells infused for posttransplant relapse.J Clin Invest. 2022 Jun 1;132(11):e154334. doi: 10.1172/JCI154334. J Clin Invest. 2022. PMID: 35349491 Free PMC article.
-
Donor Lymphocyte Infusion in the Treatment of Post-Transplant Relapse of Acute Myeloid Leukemias and Myelodysplastic Syndromes Significantly Improves Overall Survival: A French-Italian Experience of 134 Patients.Cancers (Basel). 2024 Mar 26;16(7):1278. doi: 10.3390/cancers16071278. Cancers (Basel). 2024. PMID: 38610955 Free PMC article.
References
-
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. . Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. (1990) 76:2462–5. - PubMed
-
- Dobyski WR, Keever CA, Roth MS, Koethe S, Hanson G, McFadden P, et al. Salvage immunotherapy using donor leucocyte infusions as treatment for relapsed chronic myelogenous leukemia after allogeneic bone marrow transplantation: efficacy and toxicity of a defined T-cell dose. Blood. (1993) 82:2310–8. 10.1182/blood.V82.8.2310.2310 - DOI - PubMed
-
- Verdonck LF, Petersen EJ, Lokhorst HM, Nieuwenhuis HK, Dekker AW, Tilanus MG, et al. . Donor leukocyte infusions for recurrent hematologic malignancies after allogeneic bone marrow transplantation: impact of infused and residual donor T cells. Bone Marrow Transplant. (1998) 22:1057–63. 10.1038/sj.bmt.1701496 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials