Scaffolds of Macroporous Tannin Spray With Human-Induced Pluripotent Stem Cells
- PMID: 33178667
- PMCID: PMC7593690
- DOI: 10.3389/fbioe.2020.00951
Scaffolds of Macroporous Tannin Spray With Human-Induced Pluripotent Stem Cells
Abstract
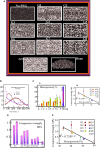
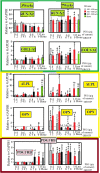
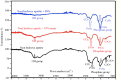
Skeletal defects resulting from trauma and disease represent a major clinical problem worldwide exacerbated further by global population growth and an increasing number of elderly people. As treatment options are limited, bone tissue engineering opens the doors to start an infinite amount of tissue/bone biomaterials having excellent therapeutic potential for the management of clinical cases characterized by severe bone loss. Bone engineering relies on the use of compliant biomaterial scaffolds, osteocompetent cells, and biologically active agents. In fact, we are interested to use a new natural material, tannin. Among other materials, porous tannin spray-dried powder (PTSDP) has been approved for human use. We use PTSDP as reconstructive materials with low cost, biocompatibility, and potential ability to be replaced by bone in vivo. In this study, macro PTSDP scaffolds with defined geometry, porosity, and mechanical properties are manufactured using a combination of casting technology and porogen leaching, by mixing PTSDP and hydroxyapatite Ca10(PO4)6(OH)2 with polyethylene glycol macroparticles. Our results show that the scaffolds developed in this work support attachment, long-term viability, and osteogenic differentiation of human-induced pluripotent stem cell-derived mesenchymal progenitors. The combination of select macroporous PTSDP scaffolds with patient-specific osteocompetent cells offers new opportunities to grow autologous bone grafts with enhanced clinical potential for complex skeletal reconstructions.
Keywords: bone engineering; macroporous tannin spray; solid scaffolds; tannin composite; vivo-stem cells.
Copyright © 2020 Yang and Abdalla.
Figures







Similar articles
-
Engineering human bone grafts with new macroporous calcium phosphate cement scaffolds.J Tissue Eng Regen Med. 2018 Mar;12(3):715-726. doi: 10.1002/term.2491. Epub 2017 Sep 25. J Tissue Eng Regen Med. 2018. PMID: 28635177
-
Fabrication of macroporous cement scaffolds using PEG particles: In vitro evaluation with induced pluripotent stem cell-derived mesenchymal progenitors.Mater Sci Eng C Mater Biol Appl. 2016 Dec 1;69:640-52. doi: 10.1016/j.msec.2016.06.075. Epub 2016 Jun 30. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27612757
-
Preparation, in vitro degradability, cytotoxicity, and in vivo biocompatibility of porous hydroxyapatite whisker-reinforced poly(L-lactide) biocomposite scaffolds.J Biomater Sci Polym Ed. 2016;27(6):505-28. doi: 10.1080/09205063.2016.1140613. Epub 2016 Feb 12. J Biomater Sci Polym Ed. 2016. PMID: 26873015
-
Macroporous scaffolds associated with cells to construct a hybrid biomaterial for bone tissue engineering.Expert Rev Med Devices. 2008 Nov;5(6):719-28. doi: 10.1586/17434440.5.6.719. Expert Rev Med Devices. 2008. PMID: 19025348 Review.
-
Silk scaffolds in bone tissue engineering: An overview.Acta Biomater. 2017 Nov;63:1-17. doi: 10.1016/j.actbio.2017.09.027. Epub 2017 Sep 20. Acta Biomater. 2017. PMID: 28941652 Review.
References
-
- Abdalla S., Al-Marzouki F., Pizzi A., Bahabri F. (2018). Bone Graft with a Tannin-hydroxyapatite Scaffold and Stem Cells for Bone Engineering, U.S. Patent number: 10155069. Washington, DC: U.S. Patent and Trademark Office.
-
- Abdalla S., Pizzi A., Bahabri F., Ganash A. (2015). MALDI-TOF and C13 NMR analysis of Valonea oak (Quercus aegylops) aicorn tannin and its adhesive application. Bioresources 10 5233–5244.
-
- Al-Marzouki F., Zahed A., Pizzi A., Abdalla S. (2017). Thermo-set Resin Composition for Brake Pads, Method of Preparation, and Brake Pad Assembly, Patent number: 9791012. Washington, DC: U.S. Patent and Trademark Office.
-
- Amaral-Labat G., Grishechko L., Szczurek A., Fierro V., Pizzi A., Kuznetsov B., et al. (2012). Highly mesoporous organic aerogels derived from soy and tannin. Green Chem. 14 3099–3106.
LinkOut - more resources
Full Text Sources
Research Materials

