Insights on the DNA Stability in Aqueous Solutions of Ionic Liquids
- PMID: 33178668
- PMCID: PMC7591794
- DOI: 10.3389/fbioe.2020.547857
Insights on the DNA Stability in Aqueous Solutions of Ionic Liquids
Abstract
Deoxyribonucleic acid (DNA) carries the genetic information essential for the growth and functioning of living organisms, playing a significant role in life sciences research. However, the long-term storage and preservation of DNA, while ensuring its bioactivity, are still current challenges to overcome. In this work, aqueous solutions of ionic liquids (ILs) were investigated as potential preservation media for double stranded (dsDNA). A screening of several ILs, by combining the cholinium, tetrabutylammonium, tetrabutylphosphonium, and 1-ethyl-3-methylimidazolium, cations with the anions bromide, chloride, dihydrogen phosphate, acetate, and glycolate, was carried out in order to gather fundamental knowledge on the molecular features of ILs that improve the dsDNA stability. Different IL concentrations and the pH effect were also addressed. Circular dichroism (CD) spectroscopy was used to evaluate the conformational structure and stability of dsDNA. IL-DNA interactions were appraised by UV-Vis absorption spectrophotometry and 31P nuclear magnetic resonance (NMR) spectroscopy. The results obtained demonstrate that pH has a significant effect towards the dsDNA stability. Amongst the ILs investigated, cholinium-based ILs are the most promising class of ILs to preserve the dsDNA structure, in which electrostatic interactions between the cholinium cation and the DNA phosphate groups play a significant role as demonstrated by the 31P NMR data, being more relevant at higher IL concentrations. On the other hand, the denaturation of dsDNA mainly occurs with ILs composed of more hydrophobic cations and able to establish dispersive interactions with the nucleobases environment. Furthermore, the IL anion has a weaker impact when compared to the IL cation effect to interact with DNA molecules. The experimental data of this work provide relevant fundamental knowledge for the application of ILs in the preservation of nucleic acids, being of high relevance in the biotechnology field.
Keywords: DNA; interactions; ionic liquids; native conformation; nucleic acid; stability.
Copyright © 2020 Dinis, Sousa and Freire.
Figures
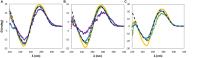
 ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [N4444]Br; (
) [N4444]Br; ( ) [C2C1im]Br; (
) [C2C1im]Br; ( ) [P4444]Br.
) [P4444]Br.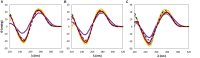
 ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [N111(2OH)]Cl; (
) [N111(2OH)]Cl; ( ) [N111(2OH)][Ac]; (
) [N111(2OH)][Ac]; ( ) non-buffered [N111(2OH)][DHP]; (
) non-buffered [N111(2OH)][DHP]; ( ) buffered [N111(2OH)][DHP]; (
) buffered [N111(2OH)][DHP]; ( ) [N111(2OH)][Gly].
) [N111(2OH)][Gly].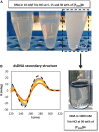
 ) only buffer; (
) only buffer; ( ) 5 wt% of [P4444]Br; (
) 5 wt% of [P4444]Br; ( ) 15 wt% of [P4444]Br; (
) 15 wt% of [P4444]Br; ( ) 30 wt% of [P4444]Br.
) 30 wt% of [P4444]Br.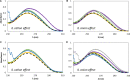
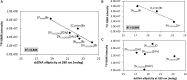
 ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [C2C1im]Br; (
) [C2C1im]Br; ( ) [N4444]Br; (
) [N4444]Br; ( ) [P4444]Br; (B) 5 wt% of cholinium-based ILs. (
) [P4444]Br; (B) 5 wt% of cholinium-based ILs. ( ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [N111(2OH)][Ac]; (
) [N111(2OH)][Ac]; ( ) non-buffered [N111(2OH)][DHP]; (
) non-buffered [N111(2OH)][DHP]; ( ) [N111(2OH)]Cl; (
) [N111(2OH)]Cl; ( ) [N111(2OH)][Gly]; (C) 30 wt% of bromide-based ILs: (
) [N111(2OH)][Gly]; (C) 30 wt% of bromide-based ILs: ( ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [C2C1im]Br; (
) [C2C1im]Br; ( ) [N4444]Br; (D) 30 wt% of cholinium-based ILs. (
) [N4444]Br; (D) 30 wt% of cholinium-based ILs. ( ) only buffer; (
) only buffer; ( ) [N111(2OH)]Br; (
) [N111(2OH)]Br; ( ) [N111(2OH)][Ac]; (
) [N111(2OH)][Ac]; ( ) non-buffered [N111(2OH)][DHP]; (
) non-buffered [N111(2OH)][DHP]; ( ) [N111(2OH)]Cl; (
) [N111(2OH)]Cl; ( ) [N111(2OH)][Gly].
) [N111(2OH)][Gly].
References
-
- Bowlas C. J., Bruce D. W., Seddon K. R. (1996). Liquid-crystalline ionic liquids. ChemComm 1996 1625–1626. 10.1039/cc9960001625 - DOI
-
- Cláudio A. F. M., Ferreira A. M., Freire M. G., Coutinho J. A. P. (2013). Enhanced extraction of caffeine from guaraná seeds using aqueous solutions of ionic liquids. Green Chem. 15 2002–2010. 10.1039/C3GC40437D - DOI
LinkOut - more resources
Full Text Sources

