Gene Expression Signatures of Synovial Fluid Multipotent Stromal Cells in Advanced Knee Osteoarthritis and Following Knee Joint Distraction
- PMID: 33178674
- PMCID: PMC7591809
- DOI: 10.3389/fbioe.2020.579751
Gene Expression Signatures of Synovial Fluid Multipotent Stromal Cells in Advanced Knee Osteoarthritis and Following Knee Joint Distraction
Abstract
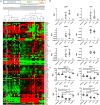
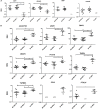
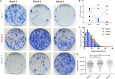
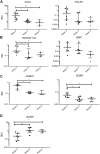
Osteoarthritis (OA) is the most common musculoskeletal disorder. Although joint replacement remains the standard of care for knee OA patients, knee joint distraction (KJD), which works by temporarily off-loading the joint for 6-8 weeks, is becoming a novel joint-sparing alternative for younger OA sufferers. The biological mechanisms behind KJD structural improvements remain poorly understood but likely involve joint-resident regenerative cells including multipotent stromal cells (MSCs). In this study, we hypothesized that KJD leads to beneficial cartilage-anabolic and anti-catabolic changes in joint-resident MSCs and investigated gene expression profiles of synovial fluid (SF) MSCs following KJD as compared with baseline. To obtain further insights into the effects of local biomechanics on MSCs present in late OA joints, SF MSC gene expression was studied in a separate OA arthroplasty cohort and compared with subchondral bone (SB) MSCs from medial (more loaded) and lateral (less loaded) femoral condyles from the same joints. In OA arthroplasty cohort (n = 12 patients), SF MSCs expressed lower levels of ossification- and hypotrophy-related genes [bone sialoprotein (IBSP), parathyroid hormone 1 receptor (PTH1R), and runt-related transcription factor 2 (RUNX2)] than did SB MSCs. Interestingly, SF MSCs expressed 5- to 50-fold higher levels of transcripts for classical extracellular matrix turnover molecules matrix metalloproteinase 1 (MMP1), a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), and tissue inhibitor of metalloproteinase-3 (TIMP3), all (p < 0.05) potentially indicating greater cartilage remodeling ability of OA SF MSCs, compared with SB MSCs. In KJD cohort (n = 9 patients), joint off-loading resulted in sustained, significant increase in SF MSC colonies' sizes and densities and a notable transcript upregulation of key cartilage core protein aggrecan (ACAN) (weeks 3 and 6), as well as reduction in pro-inflammatory C-C motif chemokine ligand 2 (CCL2) expression (weeks 3 and 6). Additionally, early KJD changes (week 3) were marked by significant increases in MSC chondrogenic commitment markers gremlin 1 (GREM1) and growth differentiation factor 5 (GDF5). In combination, our results reveal distinct transcriptomes on joint-resident MSCs from different biomechanical environments and show that 6-week joint off-loading leads to transcriptional changes in SF MSCs that may be beneficial for cartilage regeneration. Biomechanical factors should be certainly considered in the development of novel MSC-based therapies for OA.
Keywords: chondrocytes; knee joint distraction; multipotent stromal cells; osteoarthritis; subchondral bone; synovial fluid.
Copyright © 2020 Sanjurjo-Rodriguez, Altaie, Mastbergen, Baboolal, Welting, Lafeber, Pandit, McGonagle and Jones.
Figures

References
-
- Amemiya M., Tsuji K., Katagiri H., Miyatake K., Nakagawa Y., Sekiya I., et al. (2020). Synovial fluid-derived mesenchymal cells have non-inferior chondrogenic potential and can be utilized for regenerative therapy as substitute for synovium-derived cells. Biochem. Biophys. Res. Commun. 523 465–472. 10.1016/j.bbrc.2019.12.068 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

