Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies
- PMID: 33178688
- PMCID: PMC7593554
- DOI: 10.3389/fcell.2020.569219
Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies
Abstract
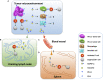
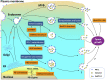
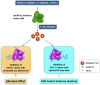
As a classical immune checkpoint molecule, PD-L1 on the surface of tumor cells plays a pivotal role in tumor immunosuppression, primarily by inhibiting the antitumor activities of T cells by binding to its receptor PD-1. PD-1/PD-L1 inhibitors have demonstrated unprecedented promise in treating various human cancers with impressive efficacy. However, a significant portion of cancer patients remains less responsive. Therefore, a better understanding of PD-L1-mediated immune escape is imperative. PD-L1 can be expressed on the surface of tumor cells, but it is also found to exist in extracellular forms, such as on exosomes. Recent studies have revealed the importance of exosomal PD-L1 (ExoPD-L1). As an alternative to membrane-bound PD-L1, ExoPD-L1 produced by tumor cells also plays an important regulatory role in the antitumor immune response. We review the recent remarkable findings on the biological functions of ExoPD-L1, including the inhibition of lymphocyte activities, migration to PD-L1-negative tumor cells and immune cells, induction of both local and systemic immunosuppression, and promotion of tumor growth. We also discuss the potential implications of ExoPD-L1 as a predictor for disease progression and treatment response, sensitive methods for detection of circulating ExoPD-L1, and the novel therapeutic strategies combining the inhibition of exosome biogenesis with PD-L1 blockade in the clinic.
Keywords: abscopal effect; antitumor immune memory; biomarker; detection method; exosomal PD-L1; immune escape; immunotherapy; tumor microenvironment.
Copyright © 2020 Zhou, Guo, Li, Sun and Liang.
Figures
Similar articles
-
Exosomal PD-L1 in cancer and other fields: recent advances and perspectives.Front Immunol. 2024 Apr 25;15:1395332. doi: 10.3389/fimmu.2024.1395332. eCollection 2024. Front Immunol. 2024. PMID: 38726017 Free PMC article. Review.
-
Clinical Implications of Exosomal PD-L1 in Cancer Immunotherapy.J Immunol Res. 2021 Feb 8;2021:8839978. doi: 10.1155/2021/8839978. eCollection 2021. J Immunol Res. 2021. PMID: 33628854 Free PMC article. Review.
-
Biological Characteristics and Clinical Significance of Soluble PD-1/PD-L1 and Exosomal PD-L1 in Cancer.Front Immunol. 2022 Mar 21;13:827921. doi: 10.3389/fimmu.2022.827921. eCollection 2022. Front Immunol. 2022. PMID: 35386715 Free PMC article. Review.
-
ExoPD-L1: an assistant for tumor progression and potential diagnostic marker.Front Oncol. 2023 Sep 5;13:1194180. doi: 10.3389/fonc.2023.1194180. eCollection 2023. Front Oncol. 2023. PMID: 37736550 Free PMC article. Review.
-
Clinical significance of circulating exosomal PD-L1 and soluble PD-L1 in extranodal NK/T-cell lymphoma, nasal-type.Am J Cancer Res. 2020 Dec 1;10(12):4498-4512. eCollection 2020. Am J Cancer Res. 2020. PMID: 33415014 Free PMC article.
Cited by
-
Tumor-Derived Extracellular Vesicles in Cancer Immunoediting and Their Potential as Oncoimmunotherapeutics.Cancers (Basel). 2022 Dec 23;15(1):82. doi: 10.3390/cancers15010082. Cancers (Basel). 2022. PMID: 36612080 Free PMC article. Review.
-
Soluble Programmed Death Ligand-1 (sPD-L1): A Pool of Circulating Proteins Implicated in Health and Diseases.Cancers (Basel). 2021 Jun 17;13(12):3034. doi: 10.3390/cancers13123034. Cancers (Basel). 2021. PMID: 34204509 Free PMC article. Review.
-
Necroptosis-related lncRNA-based novel signature to predict the prognosis and immune landscape in soft tissue sarcomas.J Cancer Res Clin Oncol. 2024 Apr 18;150(4):203. doi: 10.1007/s00432-024-05682-w. J Cancer Res Clin Oncol. 2024. PMID: 38635069 Free PMC article.
-
Dendritic Cells Pulsed with HAM/TSP Exosomes Sensitize CD4 T Cells to Enhance HTLV-1 Infection, Induce Helper T-Cell Polarization, and Decrease Cytotoxic T-Cell Response.Viruses. 2024 Sep 10;16(9):1443. doi: 10.3390/v16091443. Viruses. 2024. PMID: 39339919 Free PMC article.
-
[Research Advances of Immunotherapy of Exosome PD-L1 in Non-small Cell Lung Cancer].Zhongguo Fei Ai Za Zhi. 2022 Sep 20;25(9):689-695. doi: 10.3779/j.issn.1009-3419.2022.102.33. Zhongguo Fei Ai Za Zhi. 2022. PMID: 36172735 Free PMC article. Review. Chinese.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

