Extracellular Control of Radial Glia Proliferation and Scaffolding During Cortical Development and Pathology
- PMID: 33178693
- PMCID: PMC7596222
- DOI: 10.3389/fcell.2020.578341
Extracellular Control of Radial Glia Proliferation and Scaffolding During Cortical Development and Pathology
Abstract
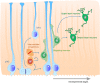
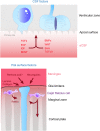
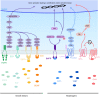
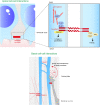
During the development of the cortex, newly generated neurons migrate long-distances in the expanding tissue to reach their final positions. Pyramidal neurons are produced from dorsal progenitors, e.g., radial glia (RGs) in the ventricular zone, and then migrate along RG processes basally toward the cortex. These neurons are hence dependent upon RG extensions to support their migration from apical to basal regions. Several studies have investigated how intracellular determinants are required for RG polarity and subsequent formation and maintenance of their processes. Fewer studies have identified the influence of the extracellular environment on this architecture. This review will focus on extracellular factors which influence RG morphology and pyramidal neuronal migration during normal development and their perturbations in pathology. During cortical development, RGs are present in different strategic positions: apical RGs (aRGs) have their cell bodies located in the ventricular zone with an apical process contacting the ventricle, while they also have a basal process extending radially to reach the pial surface of the cortex. This particular conformation allows aRGs to be exposed to long range and short range signaling cues, whereas basal RGs (bRGs, also known as outer RGs, oRGs) have their cell bodies located throughout the cortical wall, limiting their access to ventricular factors. Long range signals impacting aRGs include secreted molecules present in the embryonic cerebrospinal fluid (e.g., Neuregulin, EGF, FGF, Wnt, BMP). Secreted molecules also contribute to the extracellular matrix (fibronectin, laminin, reelin). Classical short range factors include cell to cell signaling, adhesion molecules and mechano-transduction mechanisms (e.g., TAG1, Notch, cadherins, mechanical tension). Changes in one or several of these components influencing the RG extracellular environment can disrupt the development or maintenance of RG architecture on which neuronal migration relies, leading to a range of cortical malformations. First, we will detail the known long range signaling cues impacting RG. Then, we will review how short range cell contacts are also important to instruct the RG framework. Understanding how RG processes are structured by their environment to maintain and support radial migration is a critical part of the investigation of neurodevelopmental disorders.
Keywords: apical radial glia; cell signaling; cell-cell interaction; cortical development; extracellular matrix; neuronal migration; scaffold.
Copyright © 2020 Ferent, Zaidi and Francis.
Figures

References
-
- Anton E. S., Marchionni M. A., Lee K. F., Rakic P. (1997). Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development 124 3501–3510. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

