Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration
- PMID: 33178704
- PMCID: PMC7593613
- DOI: 10.3389/fcell.2020.594226
Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration
Abstract
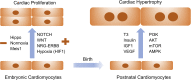
In the past few decades, cardiac regeneration has been the central target for restoring the injured heart. In mammals, cardiomyocytes are terminally differentiated and rarely divide during adulthood. Embryonic and fetal cardiomyocytes undergo robust proliferation to form mature heart chambers in order to accommodate the increased workload of a systemic circulation. In contrast, postnatal cardiomyocytes stop dividing and initiate hypertrophic growth by increasing the size of the cardiomyocyte when exposed to increased workload. Extracellular and intracellular signaling pathways control embryonic cardiomyocyte proliferation and postnatal cardiac hypertrophy. Harnessing these pathways could be the future focus for stimulating endogenous cardiac regeneration in response to various pathological stressors. Meanwhile, patient-specific cardiomyocytes derived from autologous induced pluripotent stem cells (iPSCs) could become the major exogenous sources for replenishing the damaged myocardium. Human iPSC-derived cardiomyocytes (iPSC-CMs) are relatively immature and have the potential to increase the population of cells that advance to physiological hypertrophy in the presence of extracellular stimuli. In this review, we discuss how cardiac proliferation and maturation are regulated during embryonic development and postnatal growth, and explore how patient iPSC-CMs could serve as the future seed cells for cardiac cell replacement therapy.
Keywords: cardiac regeneration; cardiac stem cell therapy; cardiomyocyte hypertrophy; cardiomyocyte maturation; cardiomyocyte proliferation; induced pluriopotent stem cells.
Copyright © 2020 Zhao, Ye, Su and Garg.
Figures

Similar articles
-
Comparison of Non-Coding RNAs in Exosomes and Functional Efficacy of Human Embryonic Stem Cell- versus Induced Pluripotent Stem Cell-Derived Cardiomyocytes.Stem Cells. 2017 Oct;35(10):2138-2149. doi: 10.1002/stem.2669. Epub 2017 Jul 31. Stem Cells. 2017. PMID: 28710827 Free PMC article.
-
New directions in strategies using cell therapy for heart disease.J Mol Med (Berl). 2003 May;81(5):288-96. doi: 10.1007/s00109-003-0432-0. Epub 2003 Apr 16. J Mol Med (Berl). 2003. PMID: 12698252 Review.
-
Role of somatic cell sources in the maturation degree of human induced pluripotent stem cell-derived cardiomyocytes.Biochim Biophys Acta Mol Cell Res. 2020 Mar;1867(3):118538. doi: 10.1016/j.bbamcr.2019.118538. Epub 2019 Aug 28. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31472168
-
Cardiomyocyte cell cycling, maturation, and growth by multinucleation in postnatal swine.J Mol Cell Cardiol. 2020 Sep;146:95-108. doi: 10.1016/j.yjmcc.2020.07.004. Epub 2020 Jul 22. J Mol Cell Cardiol. 2020. PMID: 32710980 Free PMC article.
-
Cardiac regeneration with pluripotent stem cell-derived cardiomyocytes and direct cardiac reprogramming.Regen Ther. 2019 Jun 27;11:95-100. doi: 10.1016/j.reth.2019.06.004. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31304202 Free PMC article. Review.
Cited by
-
Probing single ventricle heart defects with patient-derived induced pluripotent stem cells and emerging technologies.Birth Defects Res. 2022 Oct 1;114(16):959-971. doi: 10.1002/bdr2.1989. Epub 2022 Feb 24. Birth Defects Res. 2022. PMID: 35199491 Free PMC article. Review.
-
Atypically Shaped Cardiomyocytes (ACMs): The Identification, Characterization and New Insights into a Subpopulation of Cardiomyocytes.Biomolecules. 2022 Jun 27;12(7):896. doi: 10.3390/biom12070896. Biomolecules. 2022. PMID: 35883452 Free PMC article. Review.
-
Decoding Genetics of Congenital Heart Disease Using Patient-Derived Induced Pluripotent Stem Cells (iPSCs).Front Cell Dev Biol. 2021 Jan 21;9:630069. doi: 10.3389/fcell.2021.630069. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33585486 Free PMC article. Review.
-
MBNL1 Regulates Programmed Postnatal Switching Between Regenerative and Differentiated Cardiac States.Circulation. 2024 Jun 4;149(23):1812-1829. doi: 10.1161/CIRCULATIONAHA.123.066860. Epub 2024 Mar 1. Circulation. 2024. PMID: 38426339 Free PMC article.
-
Hapln1 promotes dedifferentiation and proliferation of iPSC-derived cardiomyocytes by promoting versican-based GDF11 trapping.J Pharm Anal. 2024 Mar;14(3):335-347. doi: 10.1016/j.jpha.2023.09.013. Epub 2023 Sep 22. J Pharm Anal. 2024. PMID: 38618242 Free PMC article.
References
-
- Belakavadi M., Saunders J., Weisleder N., Raghava P. S., Fondell J. D. (2010). Repression of cardiac phospholamban gene expression is mediated by thyroid hormone receptor-{alpha}1 and involves targeted covalent histone modifications. Endocrinology 151 2946–2956. 10.1210/en.2009-1241 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

