Comprehensive review on the prevailing COVID-19 therapeutics and the potential of repurposing SARS-CoV-1 candidate drugs to target SARS-CoV-2 as a fast-track treatment and prevention option
- PMID: 33178779
- PMCID: PMC7607133
- DOI: 10.21037/atm-20-4071
Comprehensive review on the prevailing COVID-19 therapeutics and the potential of repurposing SARS-CoV-1 candidate drugs to target SARS-CoV-2 as a fast-track treatment and prevention option
Abstract
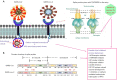
The recent seemingly uncontrollable pandemic caused by the novel severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) has been able to spread quickly due to the non-availability of effective antivirals or vaccines. The virus has structural and non-structural proteins that are considered as possible targets. Receptor recognition is the critical determinant and preliminary phase of viral infection to enter the host cell and causes tissue tropism. We have conducted a comprehensive review of relevant publication on in vitro, in silico, in vivo and clinical evaluation of drug candidates ranging from broad-spectrum antivirals to natural molecules targeted towards viral spike protein in addition to evaluate their suitability as therapies based on an analysis of the similarities between SARS-CoV-1 and SARS-CoV-2. In general, antiviral targets are based on two strategies, either targeting the host or the host's immune cell. We have reviewed the available details on the SARS-CoV-2 strain's host-viral binding sites entry mechanism, alongside recently tested effective antivirals. The hypothesis of this review may provide clear insight for researchers and physicians who are struggling to narrow down scientific options to control the current pandemic. Overall, we found that the promising efficacious drug candidates reported against SARS-CoV-1 could be considered for drug repurposing; this might help to identify a potential drug for therapeutic measures and development of vaccine for COVID-19.
Keywords: COVID-19; SARS-CoV-1; Severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2); antivirals; repurposing.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at available at http://dx.doi.org/10.21037/atm-20-4071). The authors have no conflicts of interest to declare.
Figures
References
-
- Coronavirus death report - WHO [database on the Internet] 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed: 30-6-2020.
-
- Sanche S, Lin YT, Xu C, et al. The novel coronavirus, 2019-nCoV, is highly contagious and more infectious than initially estimated. arXiv preprint arXiv:200203268 2020.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
