New solutions to old problems-metabolic acidosis in chronic kidney disease
- PMID: 33178788
- PMCID: PMC7607064
- DOI: 10.21037/atm-2020-70
New solutions to old problems-metabolic acidosis in chronic kidney disease
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-2020-70). KKZ reports personal fees from Abbott, personal fees from Abbvie, personal fees from Alexion, personal fees from Amgen, personal fees from Astra-Zeneca, personal fees from Aveo, personal fees from Chugai, other from DaVita, personal fees from Fresenius Medical Services, personal fees from Genentech, personal fees from Haymarket, personal fees from Hospira, personal fees from Kabi, personal fees from Keryx, personal fees from Navartis, personal fees from Pfizer, personal fees from Relypsa, personal fees from Resverlogix, personal fees from Sandoz, personal fees from Sanofi, personal fees from Shire, personal fees from Vifor, personal fees from ZS-Pharma, personal fees from UpToDate, grants and personal fees from National Institutes of Health, personal fees from Baxter, personal fees from Dr Schaer, personal fees from PCORI, personal fees from Amag Pharma, outside the submitted work; The other authors have no conflicts of interest to declare.
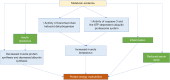
Figures
Comment on
-
Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension.Lancet. 2019 Aug 3;394(10196):396-406. doi: 10.1016/S0140-6736(19)31388-1. Epub 2019 Jun 24. Lancet. 2019. PMID: 31248662 Clinical Trial.
References
-
- KDIGO . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:136-50. - PubMed