Evaluation of diffusion-weighted MRI and (18F) fluorothymidine-PET biomarkers for early response assessment in patients with operable non-small cell lung cancer treated with neoadjuvant chemotherapy
- PMID: 33178953
- PMCID: PMC7592464
- DOI: 10.1259/bjro.20190029
Evaluation of diffusion-weighted MRI and (18F) fluorothymidine-PET biomarkers for early response assessment in patients with operable non-small cell lung cancer treated with neoadjuvant chemotherapy
Abstract
Objective: To correlate changes in the apparent diffusion coefficient (ADC) from diffusion-weighted (DW)-MRI and standardised uptake value (SUV) from fluorothymidine (18FLT)-PET/CT with histopathological estimates of response in patients with non-small cell lung cancer (NSCLC) treated with neoadjuvant chemotherapy and track longitudinal changes in these biomarkers in a multicentre, multivendor setting.
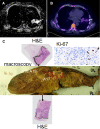
Methods: 14 patients with operable NSCLC recruited to a prospective, multicentre imaging trial (EORTC-1217) were treated with platinum-based neoadjuvant chemotherapy. 13 patients had DW-MRI and FLT-PET/CT at baseline (10 had both), 12 were re-imaged at Day 14 (eight dual-modality) and nine after completing chemotherapy, immediately before surgery (six dual-modality). Surgical specimens (haematoxylin-eosin and Ki67 stained) estimated the percentage of residual viable tumour/necrosis and proliferation index.
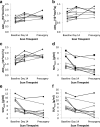
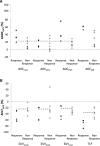
Results: Despite the small numbers,significant findings were possible. ADCmedian increased (p < 0.001) and SUVmean decreased (p < 0.001) significantly between baseline and Day 14; changes between Day 14 and surgery were less marked. All responding tumours (>30% reduction in unidimensional measurement pre-surgery), showed an increase at Day 14 in ADC75th centile and reduction in total lesion proliferation (SUVmean x proliferative volume) greater than established measurement variability. Change in imaging biomarkers did not correlate with histological response (residual viable tumour, necrosis).
Conclusion: Changes in ADC and FLT-SUV following neoadjuvant chemotherapy in NSCLC were measurable by Day 14 and preceded changes in unidimensional size but did not correlate with histopathological response. However, the magnitude of the changes and their utility in predicting (non-) response (tumour size/clinical outcome) remains to be established.
Advances in knowledge: During treatment, ADC increase precedes size reductions, but does not reflect histopathological necrosis.
© 2019 The Authors. Published by the British Institute of Radiology.
Conflict of interest statement
Conflict of Interest: John Waterton has received compensation from Bioxydyn Ltd, a for-profit company engaged in the development and provision of imaging biomarker services. All other authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures


Similar articles
-
Biomarkers for site-specific response to neoadjuvant chemotherapy in epithelial ovarian cancer: relating MRI changes to tumour cell load and necrosis.Br J Cancer. 2021 Mar;124(6):1130-1137. doi: 10.1038/s41416-020-01217-5. Epub 2021 Jan 4. Br J Cancer. 2021. PMID: 33398064 Free PMC article.
-
18F-fluorothymidine (FLT)-PET and diffusion-weighted MRI for early response evaluation in patients with small cell lung cancer: a pilot study.Eur J Hybrid Imaging. 2020 Jan 27;4(1):2. doi: 10.1186/s41824-019-0071-5. Eur J Hybrid Imaging. 2020. PMID: 34191195 Free PMC article.
-
Assessment of diffusion-weighted MRI and 18F-fluoro-deoxyglucose PET/CT in monitoring early response to neoadjuvant chemotherapy in adenocarcinoma of the esophagogastric junction.J Gastrointestin Liver Dis. 2013 Mar;22(1):45-52. J Gastrointestin Liver Dis. 2013. PMID: 23539390
-
A Phase II Study of 3'-Deoxy-3'-18F-Fluorothymidine PET in the Assessment of Early Response of Breast Cancer to Neoadjuvant Chemotherapy: Results from ACRIN 6688.J Nucl Med. 2015 Nov;56(11):1681-9. doi: 10.2967/jnumed.115.160663. Epub 2015 Sep 10. J Nucl Med. 2015. PMID: 26359256 Free PMC article. Clinical Trial.
-
Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas.Neuroimage Clin. 2018 Jul 5;20:51-60. doi: 10.1016/j.nicl.2018.07.001. eCollection 2018. Neuroimage Clin. 2018. PMID: 30069427 Free PMC article. Review.
Cited by
-
Delta-radiomics features of ADC maps as early predictors of treatment response in lung cancer.Insights Imaging. 2024 Aug 26;15(1):218. doi: 10.1186/s13244-024-01787-5. Insights Imaging. 2024. PMID: 39186132 Free PMC article.
-
Role of 3'-Deoxy-3'-[18F] Fluorothymidine Positron Emission Tomography-Computed Tomography as a Predictive Biomarker in Argininosuccinate Synthetase 1-Deficient Thoracic Cancers Treated With Pegargiminase.JTO Clin Res Rep. 2022 Jul 20;3(9):100382. doi: 10.1016/j.jtocrr.2022.100382. eCollection 2022 Sep. JTO Clin Res Rep. 2022. PMID: 36082278 Free PMC article.
-
Biomarkers for site-specific response to neoadjuvant chemotherapy in epithelial ovarian cancer: relating MRI changes to tumour cell load and necrosis.Br J Cancer. 2021 Mar;124(6):1130-1137. doi: 10.1038/s41416-020-01217-5. Epub 2021 Jan 4. Br J Cancer. 2021. PMID: 33398064 Free PMC article.
References
-
- Non-Small cell lung cancer survival rates. by Stage 2017;Available from.
-
- Balmanoukian A, Ettinger DS. Managing the patient with borderline resectable lung cancer. Oncology 2010; 24: 234–41. - PubMed
LinkOut - more resources
Full Text Sources

