Downregulation of lncRNA ZFAS1 inhibits the hallmarks of thyroid carcinoma via the regulation of miR‑302‑3p on cyclin D1
- PMID: 33179076
- PMCID: PMC7673324
- DOI: 10.3892/mmr.2020.11640
Downregulation of lncRNA ZFAS1 inhibits the hallmarks of thyroid carcinoma via the regulation of miR‑302‑3p on cyclin D1
Abstract
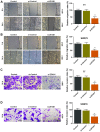
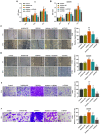
At present, treatment options for thyroid carcinoma remain limited. The present study aimed to investigate the role of ZFAS1 in various major hallmarks of cancer and the underlying mechanisms in thyroid carcinoma cells. The interactions between long non‑coding RNAs (lncRNAs), microRNAs (miRs) and target genes were predicted by bioinformatics and confirmed by performing dual‑luciferase assays. The mRNA and protein expressions were determined by reverse transcription‑quantitative PCR and western blotting. Cell invasion, migration, and viability were evaluated via Transwell, wound‑healing and Cell Counting Kit‑8 assays, respectively. The results demonstrated that lncRNA ZFAS1 expression was upregulated in thyroid carcinoma tissues, TT and SW579 cells, and was associated with the proliferation of these two cell lines. Notably, downregulation ZFAS1 reduced migration and invasion, and reversed the promotive effects of miR‑302a‑3p inhibitor on the proliferation, migration and invasion of TT and SW579 cells. Moreover, cyclin D1 (CCND1) is targeted by miR‑302a‑3p, and was regulated by ZFAS1. In addition, the downregulation of ZFAS1 not only reversed the promotive effects of miR‑302a‑3p inhibitor on CCND1 expression and the epithelial‑mesenchymal transition (EMT) of TT and SW579 cells, but also targeted and increased the expression of miR‑302a‑3p, and further reduced the expression of CCND1, resulting in suppression of the proliferation, migration, invasion and EMT of thyroid carcinoma cells.
Keywords: long non‑coding RNA ZFAS1; microRNA‑302‑3p; cyclin D1; tumorigenesis; intervention.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

