Involvement of Klotho, TNF‑α and ADAMs in radiation‑induced senescence of renal epithelial cells
- PMID: 33179086
- PMCID: PMC7673348
- DOI: 10.3892/mmr.2020.11660
Involvement of Klotho, TNF‑α and ADAMs in radiation‑induced senescence of renal epithelial cells
Abstract
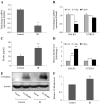
While radiation nephropathy is a major problem associated with radiotherapy, the exact mechanisms underlying its pathogenesis and the mediators involved in kidney deterioration remain to be elucidated. In view of the finding that senescence is typically increased post‑irradiation, the present study examined whether ionizing radiation may cause kidney injury by enhancing premature senescence. The present study explored the relevance of the aging suppressor, Klotho, which has anti‑aging activity and is highly expressed in murine renal cells/kidney tissues, under irradiation conditions. Firstly, the effects of radiation on mouse inner medullary collecting duct‑3 (mIMCD‑3) cells and kidney tissues of mice were assessed. Subsequently, the mRNA expression levels of Klotho, TNF‑α and ADAM metallopeptidase domain (ADAM)9/10/17 were analyzed by reverse transcription‑quantitative PCR following exposure to radiation. In addition, the levels of these proteins were measured by western blotting or ELISA. The results revealed that irradiation of mIMCD‑3 cells clearly triggered cellular senescence. Notably, Klotho gene expression was considerably decreased in radiation‑exposed mIMCD‑3 cells and in the kidney tissues of irradiated BALB/c mice, and the corresponding translated protein was consistently expressed following radiation exposure. Moreover, expression of TNF‑α, a negative regulator of Klotho, was significantly increased, whereas ADAM9/10/17, an ectodomain shedding enzyme of Klotho, was decreased in irradiated mIMCD‑3 cells and in the kidney tissues of BALB/c mice. Collectively, these data suggested that TNF‑α‑mediated inhibition of Klotho expression and blockage of soluble Klotho formation via decreased ADAM expression following irradiation may contribute to the development of renal dysfunction through acceleration of radiation‑induced cellular senescence.
Keywords: radiation; senescence; renal epithelial cells; Klotho; TNF‑α; ADAM metallopeptidase domain.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

