Dexmedetomidine attenuates sevoflurane‑induced neurocognitive impairment through α2‑adrenoceptors
- PMID: 33179100
- PMCID: PMC7684862
- DOI: 10.3892/mmr.2020.11676
Dexmedetomidine attenuates sevoflurane‑induced neurocognitive impairment through α2‑adrenoceptors
Abstract
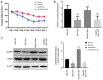
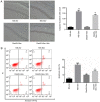
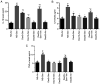
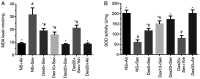
It has been reported that sevoflurane induces neurotoxicity in the developing brain. Dexmedetomidine is an α2 adrenoceptor agonist used for the prevention of sevoflurane‑induced agitation in children in clinical practice. The aim of the present study was to determine whether dexmedetomidine could prevent sevoflurane‑induced neuroapoptosis, neuroinflammation, oxidative stress and neurocognitive impairment. Additionally, the involvement of α2 adrenoceptors in the neuroprotective effect of dexmedetomidine was assessed. Postnatal day (P)6 C57BL/6 male mice were randomly divided into four groups (n=6 in each group). Mice were pretreated with dexmedetomidine, either alone or together with yohimbine, an α2 adrenoceptor inhibitor, then exposed to 3% sevoflurane in 25% oxygen. Control mice either received normal saline alone or with sevoflurane exposure. Following sevoflurane exposure, the expression of cleaved caspase‑3 was detected by immunohistochemistry in hippocampal tissue sections. In addition, the levels of tumor necrosis factor‑α (TNF‑α), interleukin (IL)‑1β, IL‑6 and malondialdehyde, as well as superoxide dismutase (SOD) activity in the hippocampus were measured. At P35, the learning and memory abilities were assessed in each mouse using a Morris water maze test. Dexmedetomidine significantly decreased the expression of activated caspase‑3 following sevoflurane exposure. Moreover, dexmedetomidine significantly decreased the levels of TNF‑α, IL‑1β and IL‑6 in the hippocampus. SOD activity also increased in a dose‑dependent manner in dexmedetomidine‑treated mice. MDA decreased in a dose‑dependent manner in dexmedetomidine‑treated mice. Lastly, sevoflurane‑induced learning and memory impairment was reversed by dexmedetomidine treatment. By contrast, co‑administration of yohimbine significantly attenuated the neuroprotective effects of dexmedetomidine. These findings suggested that dexmedetomidine exerted a neuroprotective effect against sevoflurane‑induced apoptosis, inflammation, oxidative stress and neurocognitive impairment, which was mediated, at least in part, by α2 adrenoceptors.
Keywords: dexmedetomidine; neuroapoptosis; sevoflurane; inflammation; oxidative stress; α2 adrenoceptor.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

