Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes
- PMID: 33179620
- PMCID: PMC7931572
- DOI: 10.32074/1591-951X-166
Precancerous lesions of the stomach, gastric cancer and hereditary gastric cancer syndromes
Abstract
Gastric cancer accounts for about 6% of cancers worldwide, being the fifth most frequently diagnosed malignancy and the third leading cause of cancer related death. Gastric carcinogenesis is a multistep and multifactorial process and is the result of the complex interplay between genetic susceptibility and environmental factors. The identification of predisposing conditions and of precancerous lesions is the basis for screening programs and early stage treatment. Furthermore, although most gastric cancers are sporadic, familial clustering is observed in up to 10% of patients. Among them, hereditary cases, related to known cancer susceptibility syndromes and/or genetic causes are thought to account for 1-3% of all gastric cancers. The pathology report of gastric resections specimens therefore requires a standardized approach as well as in depth knowledge of prognostic and treatment associated factors.
Keywords: gastric adenocarcinoma; gastric cancer; gastric dysplasia; hereditary diffuse gastric cancer (HDGC); hereditary gastric cancer syndromes.
Copyright © 2020 Società Italiana di Anatomia Patologica e Citopatologia Diagnostica, Divisione Italiana della International Academy of Pathology.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures
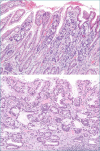
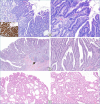
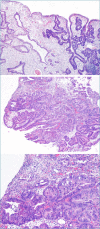
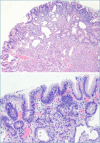


References
-
- Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. https://doi.org/10.3322/caac.21492 10.3322/caac.21492 - DOI - PubMed
-
- Gullo I, Carneiro F, Oliveira C, et al. . Heterogeneity in gastric cancer: From pure morphology to molecular classifications. Pathobiology 2018;85:50-63. https://doi.org/10.1159/000473881 10.1159/000473881 - DOI - PubMed
-
- Eusebi LH, Telese A, Marasco G, et al. . Gastric cancer prevention strategies: A global perspective. J Gastroenterol Hepatol 2020. Online ahead of print. https://doi.org/10.1111/jgh.15037 10.1111/jgh.15037 - DOI - PubMed
-
- Fukayama M, Abe H, Kunita A, et al. . Thirty years of epstein-barr virus-associated gastric carcinoma. Virchows Arch 2020;476;353-5. https://doi.org/10.1007/s00428-019-02724-4 10.1007/s00428-019-02724-4 - DOI - PubMed
-
- Sanduleanu S, Jonkers D, De Bruine A, et al. . Non-helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther 2001;15;379-88. https://doi.org/10.1046/j.1365-2036.2001.00888.x 10.1046/j.1365-2036.2001.00888.x - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

