Modeling of tumor response to macrophage and T lymphocyte interactions in the liver metastatic microenvironment
- PMID: 33180183
- PMCID: PMC10992133
- DOI: 10.1007/s00262-020-02785-4
Modeling of tumor response to macrophage and T lymphocyte interactions in the liver metastatic microenvironment
Erratum in
-
Correction to: Modeling of tumor response to macrophage and T lymphocyte interactions in the liver metastatic microenvironment.Cancer Immunol Immunother. 2021 May;70(5):1489. doi: 10.1007/s00262-020-02830-2. Cancer Immunol Immunother. 2021. PMID: 33398392 Free PMC article. No abstract available.
Abstract
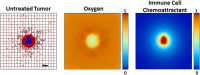
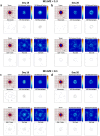
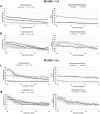
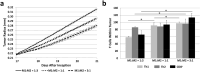
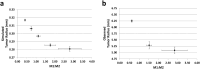
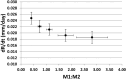
The dynamic interactions between macrophages and T-lymphocytes in the tumor microenvironment exert both antagonistic and synergistic functions affecting tumor growth. Extensive experimental effort has been expended to investigate immunotherapeutic strategies targeting macrophage polarization as well as T-cell activation with the goal to promote tumor cell killing and cancer elimination. However, these interactions remain poorly understood, and cancer immunotherapeutic strategies are often disappointing. The complex system encompassing innate and adaptive immune cell activity in response to tumor growth could benefit from a systems perspective built upon mathematical modeling. This study develops a modeling system to help evaluate the effects of macrophage and T-lymphocyte interactions on tumor growth. The system enables simulating the combined cytotoxic and tumor-promoting interactions of these two immune cell populations in a vascularized organ microenvironment, such as in liver metastases. A hypothetical immunotherapeutic strategy is simulated to increase the number of tumor-suppressive (M1-phenotype) vs. tumor-promoting (M2-phenotype) macrophages to gauge their effects on CD8+ T-cells and CD4+ T-helper cells, which in turn affect the macrophage functions. The results highlight the dynamic interactions between macrophages and T-lymphocytes in the tumor microenvironment and show that with the chosen set of parameter values, the overall cytotoxic effect from macrophages and T-lymphocytes obtained by driving the M1:M2 ratio higher could saturate and fail to achieve tumor regression. Further expansion of this modeling platform to include additional tumor-immune cell interactions, coupled with parameters representing particular tumor characteristics, could enable systematic evaluation of immunotherapeutic strategies tailored to patient-tumor specific conditions, including metastatic disease.
Keywords: Cancer immunotherapy; Cancer simulation; Liver metastasis; Mathematical modeling; T lymphocytes; Tumor-associated macrophages.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures
References
-
- Bremnes RM, Donnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6(1):209–217. doi: 10.1097/JTO.0b013e3181f8a1bd. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

