Efficacy of the AS04-adjuvanted HPV-16/18 vaccine in young Chinese women with oncogenic HPV infection at baseline: post-hoc analysis of a randomized controlled trial
- PMID: 33180670
- PMCID: PMC8018349
- DOI: 10.1080/21645515.2020.1829411
Efficacy of the AS04-adjuvanted HPV-16/18 vaccine in young Chinese women with oncogenic HPV infection at baseline: post-hoc analysis of a randomized controlled trial
Abstract
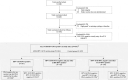
Human papillomavirus (HPV) vaccines are efficacious against HPV infections and associated lesions in women HPV-naïve at vaccination. However, vaccine efficacy (VE) against oncogenic, high-risk HPV (HR-HPV) types in women infected with any other HR-HPV type at first vaccination (baseline) remains unclear. This post-hoc analysis of a phase II/III study (NCT00779766) evaluated AS04-adjuvanted HPV-16/18 (AS04-HPV-16/18) VE against HR-HPV type infection in 871 Chinese women aged 18-25 years over a 72-month follow-up period. Study participants were DNA-negative at baseline to HR-HPV type(s) considered for VE and DNA-positive to any other HR-HPV type. Initial serostatus was not considered. Baseline DNA prevalence was 14.6% for any HR-HPV type and 10.6% excluding HPV-16/18. In the total vaccinated cohort for efficacy, VE against 6-month and 12-month HPV-16/18 persistent infections (PIs) in women DNA-negative to HPV-16/18 but DNA-positive to any other HR-HPV type at baseline was 100.0% (95% Confidence Interval [CI]: 79.8-100.0) and 100.0% (95%CI: 47.2-100.0), respectively. VE against HPV-16/18 incident infections in women DNA-positive to one vaccine type but DNA-negative to the other one at baseline was 66.8% (95%CI: -18.9-92.5). VE against HPV-31/33/45 incident infections, in women DNA-positive to HPV-16/18 and DNA-negative to the considered HPV type at baseline was 71.0% (95%CI: 27.3-89.8). No HPV-16/18 PIs were observed in vaccinated women with non-vaccine HPV A7/A9 species cervical infection at baseline. These findings indicated that women with existing HR-HPV infection at vaccination might still benefit from the AS04-HPV-16/18 vaccine. However, this potential benefit needs further demonstration in the future.
Keywords: China; Human papillomavirus; cervical cancer; clinical trial; efficacy; infection; prevention; vaccines.
Conflict of interest statement
All authors have completed the Unified Competing Interest form at
Figures
References
-
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. - DOI - PubMed
-
- Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–59. doi: 10.1016/S0140-6736(17)31821-4. - DOI - PubMed