Caspase-3 knockout attenuates radiation-induced tumor repopulation via impairing the ATM/p53/Cox-2/PGE2 pathway in non-small cell lung cancer
- PMID: 33180744
- PMCID: PMC7695367
- DOI: 10.18632/aging.103984
Caspase-3 knockout attenuates radiation-induced tumor repopulation via impairing the ATM/p53/Cox-2/PGE2 pathway in non-small cell lung cancer
Abstract
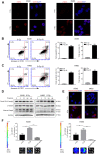
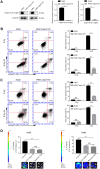
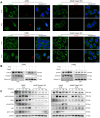
Radiotherapy is an effective treatment for non-small cell lung cancer (NSCLC). However, irradiated, dying tumor cells generate potent growth stimulatory signals during radiotherapy that promote the repopulation of adjacent surviving tumor cells to cause tumor recurrence. We investigated the function of caspase-3 in NSCLC repopulation after radiotherapy. We found that radiotherapy induced a DNA damage response (DDR), activated caspase-3, and promoted tumor repopulation in NSCLC cells. Unexpectedly, caspase-3 knockout attenuated the ataxia-telangiectasia mutated (ATM)/p53-initiated DDR by decreasing nuclear migration of endonuclease G (EndoG), thereby reducing the growth-promoting effect of irradiated, dying tumor cells. We also identified p53 as a regulator of the Cox-2/PGE2 axis and its involvement in caspase-3-induced tumor repopulation after radiotherapy. In addition, injection of caspase-3 knockout NSCLC cells impaired tumor growth in a nude mouse model. Our findings reveal that caspase-3 promotes tumor repopulation in NSCLC cells by activating DDR and the downstream Cox-2/PGE2 axis. Thus, caspase-3-induced ATM/p53/Cox-2/PGE2 signaling pathway could provide potential therapeutic targets to reduce NSCLC recurrence after radiotherapy.
Keywords: DNA damage response; caspase-3; non-small cell lung cancer; radiotherapy; tumor repopulation.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

