Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus)
- PMID: 33180820
- PMCID: PMC7660528
- DOI: 10.1371/journal.pone.0241181
Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus)
Abstract
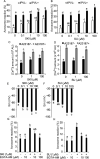
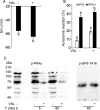
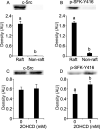
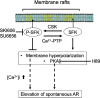
The acrosome reaction (AR) is a strictly-regulated, synchronous exocytosis that is required for sperm to penetrate ova. This all-or-nothing process occurs only once in the sperm lifecycle through a sequence of signaling pathways. Spontaneous, premature AR therefore compromises fertilization potential. Although protein kinase A (PKA) pathways play a central role in AR across species, the signaling network used for AR induction is poorly understood in birds. Mechanistic studies of mammalian sperm AR demonstrate that PKA activity is downstreamly regulated by Src family kinases (SFKs). Using SFK inhibitors, our study shows that in chicken sperm, SFKs play a role in the regulation of PKA activity and spontaneous AR without affecting motility. Furthermore, we examined the nature of SFK phosphorylation using PKA and protein tyrosine phosphatase inhibitors, which demonstrated that unlike in mammals, SFK phosphorylation in birds does not occur downstream of PKA and is primarily regulated by calcium-dependent tyrosine phosphatase activity. Functional characterization of SFKs in chicken sperm showed that SFK activation modulates the membrane potential and plays a role in inhibiting spontaneous AR. Employing biochemical isolation, we also found that membrane rafts are involved in the regulation of SFK phosphorylation. This study demonstrates a unique mechanism for regulating AR induction inherent to avian sperm that ensure fertilization potential despite prolonged storage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Membrane rafts regulate sperm acrosome reaction via cAMP-dependent pathway in chickens (Gallus gallus domesticus).Biol Reprod. 2018 Nov 1;99(5):1000-1009. doi: 10.1093/biolre/ioy120. Biol Reprod. 2018. PMID: 29788183
-
Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases.Mol Hum Reprod. 2013 Sep;19(9):570-80. doi: 10.1093/molehr/gat033. Epub 2013 Apr 29. Mol Hum Reprod. 2013. PMID: 23630234 Free PMC article.
-
SRC family kinases in hamster spermatozoa: evidence for the presence of LCK.Reproduction. 2017 May;153(5):655-669. doi: 10.1530/REP-16-0591. Epub 2017 Mar 1. Reproduction. 2017. PMID: 28250239
-
Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases.Asian J Androl. 2012 Nov;14(6):816-21. doi: 10.1038/aja.2012.81. Epub 2012 Sep 24. Asian J Androl. 2012. PMID: 23001443 Free PMC article. Review.
-
Signalling Events and Associated Pathways Related to the Mammalian Sperm Capacitation.Reprod Domest Anim. 2015 Oct;50(5):705-11. doi: 10.1111/rda.12541. Epub 2015 Aug 21. Reprod Domest Anim. 2015. PMID: 26294224 Review.
Cited by
-
A Note of Caution: Gramicidin Affects Signaling Pathways Independently of Its Effects on Plasma Membrane Conductance.Biomed Res Int. 2021 Oct 21;2021:2641068. doi: 10.1155/2021/2641068. eCollection 2021. Biomed Res Int. 2021. PMID: 34722759 Free PMC article.
-
Sperm Incubation in Biggers-Whitten-Whittingham Medium Induces Capacitation-Related Changes in the Lizard Sceloporus torquatus.Animals (Basel). 2024 May 6;14(9):1388. doi: 10.3390/ani14091388. Animals (Basel). 2024. PMID: 38731392 Free PMC article.
-
Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction.Int J Mol Sci. 2023 Nov 30;24(23):17005. doi: 10.3390/ijms242317005. Int J Mol Sci. 2023. PMID: 38069328 Free PMC article. Review.
-
Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells.Int J Mol Sci. 2023 Mar 9;24(6):5256. doi: 10.3390/ijms24065256. Int J Mol Sci. 2023. PMID: 36982326 Free PMC article. Review.
-
Membrane-Mediated Regulation of Sperm Fertilization Potential in Poultry.J Poult Sci. 2022 Apr 25;59(2):114-120. doi: 10.2141/jpsa.0210104. J Poult Sci. 2022. PMID: 35528376 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

