In-situ proliferation contributes to the accumulation of myeloid cells in the spleen during progressive experimental visceral leishmaniasis
- PMID: 33180876
- PMCID: PMC7660562
- DOI: 10.1371/journal.pone.0242337
In-situ proliferation contributes to the accumulation of myeloid cells in the spleen during progressive experimental visceral leishmaniasis
Abstract
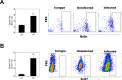
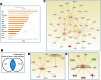
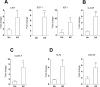
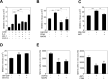
Visceral leishmaniasis (VL) is characterized by expansion of myeloid cells in the liver and spleen, which leads to a severe splenomegaly associated with higher risk of mortality. This increased cellularity is thought to be a consequence of recruitment of cells to the viscera. We studied whether the local proliferation of splenic myeloid cells contributes to increased splenic cellularity. We found that a monocyte-like population of adherent splenic cells from Leishmania donovani-infected hamsters had enhanced replicative capacity ex vivo and in vivo (BrdU incorporation, p<0.0001). In vitro assays demonstrated that proliferation was more pronounced in the proinflammatory M1 environment and that intracellular infection prevented proliferation. Secondary analysis of the published splenic transcriptome in the hamster model of progressive VL revealed a gene expression signature that included division of tumoral cells (Z = 2.0), cell cycle progression (Z = 2.3), hematopoiesis (Z = 2.8), proliferation of stem cells (Z = 2.5) and overexpression of proto-oncogenes. Regulators of myeloid cell proliferation were predicted in-silico (CSF2, TLR4, IFNG, IL-6, IL-4, RTK signaling, and STAT3). The in-silico prediction was confirmed with chemical inhibitors of PI3K/AKT, MAPK and STAT3 which decreased splenic myeloid cell division ex vivo. Hamsters infected with L. donovani treated with a STAT3 inhibitor had reduced in situ splenic myeloid proliferation (p = 0.03) and parasite burden. We conclude that monocyte-like myeloid cells have increased STAT3-dependent proliferation in the spleen of hamsters with visceral leishmaniasis and that inhibition of STAT3 reduces myeloid cell proliferation and parasite burden.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

