Chemical targeting of NEET proteins reveals their function in mitochondrial morphodynamics
- PMID: 33180995
- PMCID: PMC7726809
- DOI: 10.15252/embr.201949019
Chemical targeting of NEET proteins reveals their function in mitochondrial morphodynamics
Abstract
Several human pathologies including neurological, cardiac, infectious, cancerous, and metabolic diseases have been associated with altered mitochondria morphodynamics. Here, we identify a small organic molecule, which we named Mito-C. Mito-C is targeted to mitochondria and rapidly provokes mitochondrial network fragmentation. Biochemical analyses reveal that Mito-C is a member of a new class of heterocyclic compounds that target the NEET protein family, previously reported to regulate mitochondrial iron and ROS homeostasis. One of the NEET proteins, NAF-1, is identified as an important regulator of mitochondria morphodynamics that facilitates recruitment of DRP1 to the ER-mitochondria interface. Consistent with the observation that certain viruses modulate mitochondrial morphogenesis as a necessary part of their replication cycle, Mito-C counteracts dengue virus-induced mitochondrial network hyperfusion and represses viral replication. The newly identified chemical class including Mito-C is of therapeutic relevance for pathologies where altered mitochondria dynamics is part of disease etiology and NEET proteins are highlighted as important therapeutic targets in anti-viral research.
Keywords: NEET proteins; contact sites; mitochondria; morphodynamics; virus.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
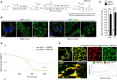
Reaction scheme for synthesis of Mito‐C and structure of the compound.
HeLa cells were treated with DMSO or 2 µM Mito‐C for 15 min and immunostained with anti‐TOM20 (green) antibody and DAPI; cropped areas show the mitochondria morphology changes.
Quantification of mitochondria morphology (based on TOM20 immunostaining as shown in B) from HeLa cells treated with DMSO or 2 µM Mito‐C for 15 min or 2 h, expressed as ratio of fragmented mitochondria (percentage of total). Errors bars show the standard error of the mean (SEM) (n = 100, technical replicates).
Profile of [2Fe–2S] cluster release from recombinant NAF‐1 (expressed and purified according to the procedure described in (Conlan et al, 2009)) was determined in untreated control (DMSO) or in presence of Mito‐C by monitoring absorbance at 458 nm as a function of time. Errors bars show the standard deviation (SD) of 3 independent experiments.
Time‐lapse video‐microscopy snapshot on HeLa cells transfected with NAF‐1‐mRFP (red) and treated with fluoMito‐C (green). The distance/intensity fluorescence quantification graph illustrates the codistribution of fluoMito‐C and NAF‐1‐mRFP.
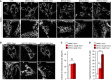
HeLa cells were treated with 2 µM Mito‐C for the indicated times, fixed and immunostained for TOM20, and analyzed by light microscopy.
HeLa cells were treated with increasing concentrations of Mito‐C for 15 min, fixed and immunostained for TOM20, and analyzed by light microscopy.
Quantification of mitochondria number (based on TOM20 immunostaining as shown in b, n = 30). Errors bars show the standard error of the mean (SEM).
Quantification of mitochondria phenotype morphology (based on TOM20 immunostaining as shown in b, n = 45–50). Errors bars show the standard error of the mean (SEM).
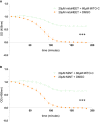
Profile of [2Fe–2S] clusters release from purified recombinant MitoNEET and MiNT were determined in presence or absence (DMSO) of Mito‐C via monitoring their absorbance at 458 nm as a function of time. Errors bars show the standard deviation (SD) of 3 independent experiments.
HeLa cells were treated with 2 µM Mito‐C or DMSO for 24 h. Cells were fixed and prepared for electron microscopy analyses (left panel). Intact mitochondrial cristae were quantified (right panel). Errors bars show the standard deviation (SD) of 3 independent experiments.
Oxygen consumption rate (Viard et al.) of cells treated with Mito‐C (2, 10 and 20µM) were measured by Seahorse© technique. Measurements start before starting the treatment, arrow indicates the Mito‐C injection. Graphs show the standard deviation (SD) of 3 independent experiments.
To evaluate the mitochondrial potential, cells were treated with Mito‐C for 24 h at the indicated range of concentrations and stained with MitoTracker Redox and analyzed by cytometry. Graphs show the standard deviation (SD) of 3 independent experiments.
To evaluate the mitochondrial potential, cells were treated with Mito‐C at 2 µM over a time course and then stained with MitoTracker Redox and analyzed by cytometry. Graphs show the standard deviation (SD) of 3 independent experiments.
To evaluate the total mitochondrial mass over a time course of treatment, HeLa cells were treated with DMSO or Mito‐C at 2 µM for the time duration indicated, stained with MitoTracker green and analyzed by cytometry. Graphs show the standard deviation (SD) of 3 independent experiments.
To evaluate the total mitochondrial mass at a fixed time point following treatment with an increasing range of concentrations of Mito‐C cells were treated with DMSO or Mito‐C for 24 h at concentration indicated, stained with MitoTracker green and analyzed by cytometry. Graphs show the standard deviation (SD) of 3 independent experiments.
High‐resolution respirometry (HRR) evaluation of HeLa cells treated for 15 min with 2 µM Mito‐C. Different bioenergetics parameters were analyzed: routine respiration, oligomycin‐sensitive, and CCCP‐sensitive. The term "routine" respiration is defined as the respiratory rate of intact cells measured in 5 mM glucose DMEM under atmospheric conditions at 37°C and sensitive to 2.5 µM antimycin A inhibition. The term "oligo insensitive" respiration is the respiratory rate measured in the routine conditions after addition of the F1F0‐ATP synthase inhibitor oligomycin at 20 µg/ml. This "oligomycin" state of respiration does not depend on ADP phosphorylation. The ATP‐linked respiration is calculated as the difference between the routine and the oligomycin‐sensitive. The term "uncoupled" respiration defines the rate of respiration measured in the "oligo" conditions after addition of the uncoupler CCCP used at 2 µM. The "CCCP" state allows evaluating the maximal capacity of the respiratory chain in presence of energy substrates and oxygen concentration as defined in the "routine" conditions. The ATP‐linked respiration (routine‐oligo) indicates the part of respiration used for ATP synthesis and the spare respiratory capacity (CCCP‐routine) gives a measure of the capacity of the respiratory chain to be chemically uncoupled. It indicates how far from the maximal capacity the routine respiration operates. Bars show the standard deviation (SD) of 3 independent experiments.
Comparison of cell viability from HeLa cells grown on high glucose (25 mM) (open bar) versus cells grown on galactose (10 mM) (solid bar) treated with 2 µM Mito‐C for 24 h. Data are expressed as mean ± SEM of N = 3 biological replicates.

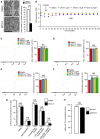
Western blot analysis of MitoNEET expression in HeLa cells transfected with CISD1 siRNA; Bar chart (right panel) shows replicates quantification of MitoNEET expression. Errors bars show the standard deviation (SD) of 3 independent experiments.
Western blot analysis of NAF‐1 expression in HeLa cells transfected with CISD2 siRNA; graph (right panel) shows the replicates quantification of NAF‐1 expression. Errors bars show the standard deviation (SD) of 3 independent experiments.
RT–qPCR analysis of CISD3 mRNA levels from HeLa cells transfected with siRNA targeting CISD3. Errors bars show the standard deviation (SD) of 9 independent experiments.
HeLa cells transfected with siRNA to reduce expression of MitoNEET (CISD1), NAF‐1 (CISD2), or MiNT (CISD3) and immunostained with anti TOM20 antibody. Arrowheads indicate fragmented mitochondria.
Quantification of mitochondria morphology (based on TOM20 immunostaining as shown in D) from HeLa cells transfected with siRNA targeting mitoNEET (siCISD1), NAF1 (siCISD2), or MiNT (siCISD3). Quantification is expressed as ratio of tubular and fragmented mitochondria. Errors bars show the standard error of the mean (SEM). (n = 125–130 cells).
Electron microscopy pictures from 150 INS‐1 cells transfected as described in G.
Western blot analysis of NAF‐1 expression in cells stably transfected with control shRNA, shRNA‐targeting reduction in NAF‐1 protein expression (shCISD2), or shRNA‐targeting reduction in NAF‐1 protein expression complemented with plasmid derived expression of NAF‐1 (shCISD2 + CISD2); Western blot quantification of NAF‐1 is shown on the accompanying bar chart. Errors bars show the standard deviation (SD) of 3 independent experiments.
Quantification of mitochondrial length in EM images from cells transfected as described in G. Errors bars show the standard error of the mean (SEM) (n = 260–270, technical replicates).
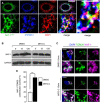
HeLa cells were transfected with NAF‐1‐GFP plasmid (green) and immunostained with antibodies to PTPIP51 (Blue) and DRP1 (red); three color merged image is shown in the far‐right panel with arrowheads indicating the white triple colocalization domains.
Western blot analysis of NAF‐1 and GAPDH proteins expression in total extracts from HeLa cells treated with 2 µM Mito‐C or DMSO for the indicated times.
HeLa cells were treated with DMSO or 2 µM Mito‐C for 15 min and immunostained with anti‐TOM20 (green) and anti‐NAF‐1 (fuchsia) antibodies and DAPI (Blue).
Quantification of NAF‐1 signal on TOM20 positive structures. Errors bars show the standard error of the mean (SEM) (n = 75 cells from 3 independent experiments).

HeLa cells treated or not, with 2 µM Mito‐C for 15 min were immunostained with anti‐TOM20 (red) and anti‐DRP1 (green); arrowheads in the far‐right panel indicate recruitment of DRP1 onto the mitochondrial surface (TOM20).
Quantification of DRP1 signal on TOM20 positive structures. Errors bars show the standard error of the mean (SEM) (n = 45, technical replicates).
Western blot analysis of DRP1, GAPDH (cytosolic marker), and TOM20 (mitochondrial marker) protein in cytosolic and mitochondrial fractions treated or not, with 2 µM Mito‐C as indicated.
Quantification of Western blots showed in C and expressed as a distribution of DRP1 in the cytosolic and mitochondrial fractions. Errors bars show the standard deviation (SD) of 5 independent experiments.
HeLa cells were transfected with DRP1K38A mutant and mCherry and treated or not, with Mito‐C (T for transfected, NT for not transfected).
Quantification of the mitochondrial phenotypes observed in E. Errors bars show the standard error of the mean (SEM) (30–35 images each with an average of 15–20 cells from triplicate independent experiments were analyzed).
Electron micrographs (EM) from HeLa cells treated with 2 µM Mito‐C or DMSO for 120 min. Empty arrowheads indicate ER‐mitochondria contact sites.
Quantification of ER‐mitochondria membrane contact‐sites events (reported in 50 µm2 section; 50 images from an average of 30 cells from triplicate independent experiments were analyzed). Errors bars show the standard error of the mean (SEM).
Quantification of ER‐mitochondria membrane contact‐sites density (in µm; 30 images from an average of 30 cells from triplicate independent experiments were analyzed). Errors bars show the standard error of the mean (SEM).
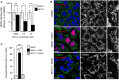
Huh7 cells were treated with Mito‐C at the indicated concentrations and simultaneously infected with dengue virus (MOI = 5). Infectious titers are presented as % of DMSO in Mito‐C concentration of 0.625, 2.5 and 10 µM conditions, upon 48 h of viral infection. Errors bars show the standard error of the mean (SEM) (n = 3).
Huh7 cells were treated with or without (DMSO) Mito‐C, infected with dengue virus and fixed after 48 h of infection. Cells were then immunostained for endogenous TOM20 (green channel), viral non‐structural protein NS5 (red) and DAPI (Blue). Cropped areas illustrate the mitochondrial morphology in described conditions.
Quantification of the mitochondrial phenotypes observed in b. Errors bars show the standard error of the mean (SEM) (15–20 images each with an average of 20–25 cells from triplicate independent experiments were analyzed).
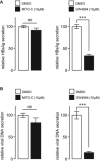
Relative secretion of HBsAg (from Hepatitis B virus (HBV)) in dHepaRG cells treated post‐infection with 10 µM of Mito‐C or the FXR agonist GW4064. Bars show the standard deviation (SD) of 3 independent experiments.
Relative secretion of HBV viral DNA in dHepaRG cells treated post‐infection with 10 µM of Mito‐C or GW4064 and quantified by quantitative PCR. Bars show the standard deviation (SD) of 3 independent experiments.
References
Publication types
MeSH terms
Substances
Grants and funding
- Z01 NR000015/ImNIH/Intramural NIH HHS/United States
- 16CONV30UPDE/Deutsche Forschungsgemeinschaft
- ANR0015TD/Agence Nationale de la Recherche
- U1151SE19KA/Deutsche Forschungsgemeinschaft
- BSF 2015831/United States - Israel Binational Science Foundation (BSF)
- 2015831/Deutsche Forschungsgemeinschaft
- 240245660/Deutsche Forschungsgemeinschaft
- L17V22CONV02/Deutsche Forschungsgemeinschaft
- U115120K/Deutsche Forschungsgemeinschaft
- ATIP-Avenir/Inserm | Inserm Transfert (Inserm Transfert SA)
- Université de Paris L17V22CONV02/Université de Paris
- Université de Paris 16CONV30UPDE/Université de Paris
- 240245660 SFB 1129TP13/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

