A Functional Topographic Map for Spinal Sensorimotor Reflexes
- PMID: 33181065
- PMCID: PMC7790959
- DOI: 10.1016/j.neuron.2020.10.003
A Functional Topographic Map for Spinal Sensorimotor Reflexes
Abstract
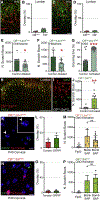
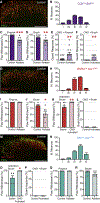
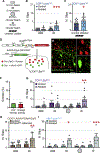
Cutaneous somatosensory modalities play pivotal roles in generating a wide range of sensorimotor behaviors, including protective and corrective reflexes that dynamically adapt ongoing movement and posture. How interneurons (INs) in the dorsal horn encode these modalities and transform them into stimulus-appropriate motor behaviors is not known. Here, we use an intersectional genetic approach to functionally assess the contribution that eight classes of dorsal excitatory INs make to sensorimotor reflex responses. We demonstrate that the dorsal horn is organized into spatially restricted excitatory modules composed of molecularly heterogeneous cell types. Laminae I/II INs drive chemical itch-induced scratching, laminae II/III INs generate paw withdrawal movements, and laminae III/IV INs modulate dynamic corrective reflexes. These data reveal a key principle in spinal somatosensory processing, namely, sensorimotor reflexes are driven by the differential spatial recruitment of excitatory neurons.
Keywords: Corrective Reflexes; Innate Behavior; Itch; Protective Reflexes; Sensorimotor Integration; Somatosensation; Somatosensory coding; Spinal Circuits; Touch.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Comment in
-
Sensory Symphonies: How Excitatory Spinal Cord Modules Orchestrate Behavior.Neuron. 2021 Jan 6;109(1):3-5. doi: 10.1016/j.neuron.2020.12.012. Neuron. 2021. PMID: 33412095 Free PMC article.
References
-
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, and Lechner SG (2017). Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

