Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain
- PMID: 33181192
- PMCID: PMC7969397
- DOI: 10.1016/j.pharmthera.2020.107743
Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain
Abstract
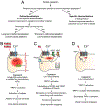
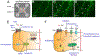
Capsaicin, the pungent ingredient in chili peppers, produces intense burning pain in humans. Capsaicin selectively activates the transient receptor potential vanilloid 1 (TRPV1), which is enriched in nociceptive primary afferents, and underpins the mechanism for capsaicin-induced burning pain. Paradoxically, capsaicin has long been used as an analgesic. The development of topical patches and injectable formulations containing capsaicin has led to application in clinical settings to treat chronic pain conditions, such as neuropathic pain and the potential to treat osteoarthritis. More detailed determination of the neurobiological mechanisms of capsaicin-induced analgesia should provide the logical rationale for capsaicin therapy and help to overcome the treatment's limitations, which include individual differences in treatment outcome and procedural discomfort. Low concentrations of capsaicin induce short-term defunctionalization of nociceptor terminals. This phenomenon is reversible within hours and, hence, likely does not account for the clinical benefit. By contrast, high concentrations of capsaicin lead to long-term defunctionalization mediated by the ablation of TRPV1-expressing afferent terminals, resulting in long-lasting analgesia persisting for several months. Recent studies have shown that capsaicin-induced Ca2+/calpain-mediated ablation of axonal terminals is necessary to produce long-lasting analgesia in a mouse model of neuropathic pain. In combination with calpain, axonal mitochondrial dysfunction and microtubule disorganization may also contribute to the longer-term effects of capsaicin. The analgesic effects subside over time in association with the regeneration of the ablated afferent terminals. Further determination of the neurobiological mechanisms of capsaicin-induced analgesia should lead to more efficacious non-opioidergic analgesic options with fewer adverse side effects.
Keywords: Analgesia; Calpain; Capsaicin; Microtubule; Neuropathic pain; Osteoarthritis; Resiniferatoxin; TRPV1.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement
James N. Campbell is on the Board of Directors and is an employee of Centrexion Therapeutics. Centrexion is involved in the commercial development of capsaicin for the treatment of pain. The other authors have no conflicts of interest to declare.
Figures
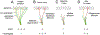
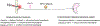
References
-
- Aasvang EK, Hansen JB, Malmstrom J, Asmussen T, Gennevois D, Struys MM, Kehlet H (2008) The effect of wound instillation of a novel purified capsaicin formulation on postherniotomy pain: a double-blind, randomized, placebo-controlled study. Anesth Analg 107:282–291. - PubMed
-
- Abooj M, Bishnoi M, Bosgraaf CA, Premkumar LS (2016) Changes in Spinal Cord Following Inflammatory and Neuropathic Pain and the Effectiveness of Resiniferatoxin. Open Pain J 9:1–4.
-
- Altman RD, Aven A, Holmburg CE, Pfeifer LM, Sack M, Young GT (1994) Capsaicin cream 0.025% as monotherapy for osteoarthritis: a double-blind study. Seminars in arthritis and rheumatism. Seminars in Arthritis and Rheumatism 23:25–33.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

