Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study
- PMID: 33181320
- PMCID: PMC7833425
- DOI: 10.1016/j.phrs.2020.105290
Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study
Abstract
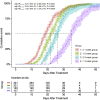
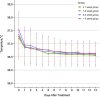
The coronavirus disease 2019 (COVID-19) epidemic has been almost controlled in China under a series of policies, including "early diagnosis and early treatment". This study aimed to explore the association between early treatment with Qingfei Paidu decoction (QFPDD) and favorable clinical outcomes. In this retrospective multicenter study, we included 782 patients (males, 56 %; median age 46) with confirmed COVID-19 from 54 hospitals in nine provinces of China, who were divided into four groups according to the treatment initiation time from the first date of onset of symptoms to the date of starting treatment with QFPDD. The primary outcome was time to recovery; days of viral shedding, duration of hospital stay, and course of the disease were also analyzed. Compared with treatment initiated after 3 weeks, early treatment with QFPDD after less than 1 week, 1-2 weeks, or 2-3 weeks had a higher likelihood of recovery, with adjusted hazard ratio (HR) (95 % confidence interval [CI]) of 3.81 (2.65-5.48), 2.63 (1.86-3.73), and 1.92 (1.34-2.75), respectively. The median course of the disease decreased from 34 days to 24 days, 21 days, and 18 days when treatment was administered early by a week (P < 0.0001). Treatment within a week was related to a decrease by 1-4 days in the median duration of hospital stay compared with late treatment (P<0.0001). In conclusion, early treatment with QFPDD may serve as an effective strategy in controlling the epidemic, as early treatment with QFPDD was associated with favorable outcomes, including faster recovery, shorter time to viral shedding, and a shorter duration of hospital stay. However, further multicenter, prospective studies with a larger sample size should be conducted to confirm the benefits of early treatment with QFPDD.
Keywords: COVID-19; Early treatment; Qingfei Paidu decoction.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures


References
-
- World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. 2020-03-11. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re... (’accessed’ May 18th 2020).
-
- World Health Organization. Statement on the third meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of coronavirus disease (COVID-19). 2020-05-01. https://www.who.int/news-room/detail/01-05-2020-statement-on-the-third-m... (’accessed’ May 18th 2020).
-
- World Health Organization . 2020. Coronavirus Disease (COVID-19) Situation Report-153; pp. 06–21.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (’Accessed’ June 21th 2020)
-
- Escalera-Antezana J.P., Lizon-Ferrufino N.F., Maldonado-Alanoca A., Alarcón-De-la-Vega G., Alvarado-Arnez L.E., Balderrama-Saavedra M.A. Clinical features of the first cases and a cluster of Coronavirus Disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Travel Med. Infect. Di. 2020;35:101653. doi: 10.1016/j.tmaid.2020.101653. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

