Hemoperfusion with CytoSorb as Adjuvant Therapy in Critically Ill Patients with SARS-CoV2 Pneumonia
- PMID: 33181508
- PMCID: PMC7705939
- DOI: 10.1159/000511725
Hemoperfusion with CytoSorb as Adjuvant Therapy in Critically Ill Patients with SARS-CoV2 Pneumonia
Erratum in
-
Erratum.Blood Purif. 2024;53(10):849. doi: 10.1159/000540847. Epub 2024 Aug 27. Blood Purif. 2024. PMID: 39191234 Free PMC article. No abstract available.
Abstract
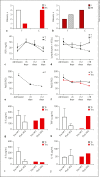
We report a preliminary experience of adjuvant therapy with Hemoperfusion (HP) in patients with Severe Acute Respiratory Syndrome-CoronaVirus 2 (SARS-CoV2) pneumonia. Currently, there are no approved treatments for CoronaVirus Disease 19 (COVID-19); however, therapeutic strategies based on the preclinical evidence include supportive measures, such as oxygen supplementation, antiviral, and anticoagulant agents. Despite these treatments, 10% of patients worsen and develop severe acute respiratory distress syndrome (ARDS). Since the pathogenic mechanism of ARDS is an uncontrolled inflammatory state, we speculate that removing inflammation effectors from blood may contrast tissue injury and improve clinical outcome. In a scenario of dramatic medical emergency, we conducted an observational study on 9 consecutive patients hospitalized in COVID Intensive Care Unit, where 5 of 9 consecutive patients were treated with HP, due to the emergency overload made it impossible to deliver blood purification in the other 4 patients. COVID-19 was diagnosed through the identification of virus sequences by reverse transcription-PCR on respiratory specimens. All patients had severe pneumonia requiring continuous positive airway pressure. HP was started in all patients 6-7 days after hospital admission. The treated patients (T) received 2 consecutive sessions of HP using CytoSorb cartridge. Our results show a better clinical course of T compared to control patients (C), in fact all T except 1 survived, and only 2 of them were intubated, while all C required intubation and died. Lymphocytopenia worsened in C but not in T. C-reactive protein decreased in both patients, but to a greater extent in T. IL-6, IL-8, and TNF-α decreased after HP, IL-10 did not change. Respiratory function remained stable and did not worsen in T compared to C. The limited sample size and observational study design preclude a sound statement about the potential effectiveness of HP in COVID-19 patients, but our experience suggests a potential therapeutic role of adjuvant CytoSorb HP in the early course of CO-VID-19 pneumonia. A randomized clinical trial is ongoing.
Keywords: Blood purification; COVID-19; Case report; Critical illness cytokines; Hemoperfusion.
The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

