HCN channels in the mammalian cochlea: Expression pattern, subcellular location, and age-dependent changes
- PMID: 33181864
- PMCID: PMC7839784
- DOI: 10.1002/jnr.24754
HCN channels in the mammalian cochlea: Expression pattern, subcellular location, and age-dependent changes
Abstract
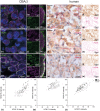
Neuronal diversity in the cochlea is largely determined by ion channels. Among voltage-gated channels, hyperpolarization-activated cyclic nucleotide-gated (HCN) channels open with hyperpolarization and depolarize the cell until the resting membrane potential. The functions for hearing are not well elucidated and knowledge about localization is controversial. We created a detailed map of subcellular location and co-expression of all four HCN subunits across different mammalian species including CBA/J, C57Bl/6N, Ly5.1 mice, guinea pigs, cats, and human subjects. We correlated age-related hearing deterioration in CBA/J and C57Bl/6N with expression levels of HCN1, -2, and -4 in individual auditory neurons from the same cohort. Spatiotemporal expression during murine postnatal development exposed HCN2 and HCN4 involvement in a critical phase of hair cell innervation. The huge diversity of subunit composition, but lack of relevant heteromeric pairing along the perisomatic membrane and axon initial segments, highlighted an active role for auditory neurons. Neuron clusters were found to be the hot spots of HCN1, -2, and -4 immunostaining. HCN channels were also located in afferent and efferent fibers of the sensory epithelium. Age-related changes on HCN subtype expression were not uniform among mice and could not be directly correlated with audiometric data. The oldest mice groups revealed HCN channel up- or downregulation, depending on the mouse strain. The unexpected involvement of HCN channels in outer hair cell function where HCN3 overlaps prestin location emphasized the importance for auditory function. A better understanding may open up new possibilities to tune neuronal responses evoked through electrical stimulation by cochlear implants.
Keywords: HCN channels; RRID:AB_2039906; RRID:AB_2302038; RRID:AB_2313584; RRID:AB_2313726; RRID:AB_2336419; RRID:AB_2336420; RRID:AB_2336790; RRID:AB_2340452; RRID:AB_2340477; RRID:AB_2340593; RRID:AB_2341028; RRID:AB_2617143; RRID:AB_2756625; RRID:AB_2756742; RRID:AB_90725; RRID:SCR_002865; RRID:SCR_013652; RRID:SCR_014823; auditory development; auditory neuron diversity; axon initial segment; prestin; sound coding; spiral ganglion neurons; voltage gated.
© 2020 The Authors. Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
There is no conflict of interest to declare.
Figures











Similar articles
-
Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea.J Neurophysiol. 2010 May;103(5):2532-43. doi: 10.1152/jn.00506.2009. Epub 2010 Mar 10. J Neurophysiol. 2010. PMID: 20220080 Free PMC article.
-
Deletion of the hyperpolarization-activated cyclic nucleotide-gated channel auxiliary subunit TRIP8b impairs hippocampal Ih localization and function and promotes antidepressant behavior in mice.J Neurosci. 2011 May 18;31(20):7424-40. doi: 10.1523/JNEUROSCI.0936-11.2011. J Neurosci. 2011. PMID: 21593326 Free PMC article.
-
The upregulation of K+ and HCN channels in developing spiral ganglion neurons is mediated by cochlear inner hair cells.J Physiol. 2024 Oct;602(20):5329-5351. doi: 10.1113/JP286134. Epub 2024 Sep 26. J Physiol. 2024. PMID: 39324853
-
Pathophysiology of HCN channels.Pflugers Arch. 2007 Jul;454(4):517-22. doi: 10.1007/s00424-007-0224-4. Epub 2007 Feb 14. Pflugers Arch. 2007. PMID: 17549513 Review.
-
Cortical HCN channels: function, trafficking and plasticity.J Physiol. 2014 Jul 1;592(13):2711-9. doi: 10.1113/jphysiol.2013.270058. Epub 2014 Apr 22. J Physiol. 2014. PMID: 24756635 Free PMC article. Review.
Cited by
-
Na/K-ATPase Gene Expression in the Human Cochlea: A Study Using mRNA in situ Hybridization and Super-Resolution Structured Illumination Microscopy.Front Mol Neurosci. 2022 Mar 31;15:857216. doi: 10.3389/fnmol.2022.857216. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35431803 Free PMC article.
-
Selective Vulnerability of GABAergic Inhibitory Interneurons to Bilirubin Neurotoxicity in the Neonatal Brain.J Neurosci. 2024 Nov 6;44(45):e0442242024. doi: 10.1523/JNEUROSCI.0442-24.2024. J Neurosci. 2024. PMID: 39313321 Free PMC article.
-
A comprehensive review of HCN channel expression and Ih in the auditory system: then, now, and future perspectives.J Neurophysiol. 2025 Aug 1;134(2):458-470. doi: 10.1152/jn.00602.2024. Epub 2025 Jul 7. J Neurophysiol. 2025. PMID: 40623052 Free PMC article. Review.
-
Closing the Gap between the Auditory Nerve and Cochlear Implant Electrodes: Which Neurotrophin Cocktail Performs Best for Axonal Outgrowth and Is Electrical Stimulation Beneficial?Int J Mol Sci. 2023 Jan 19;24(3):2013. doi: 10.3390/ijms24032013. Int J Mol Sci. 2023. PMID: 36768339 Free PMC article.
-
Spike Generators and Cell Signaling in the Human Auditory Nerve: An Ultrastructural, Super-Resolution, and Gene Hybridization Study.Front Cell Neurosci. 2021 Mar 16;15:642211. doi: 10.3389/fncel.2021.642211. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33796009 Free PMC article.
References
-
- Bakondi, G. , Por, A. , Kovacs, I. , Szucs, G. , & Rusznak, Z. (2009). Hyperpolarization‐activated, cyclic nucleotide‐gated, cation non‐selective channel subunit expression pattern of guinea‐pig spiral ganglion cells. Neuroscience, 158(4), 1469–1477. 10.1016/j.neuroscience.2008.10.056 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

