In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber
- PMID: 33182247
- PMCID: PMC7664926
- DOI: 10.3390/molecules25215201
In Vitro Prebiotic Effects of Malto-Oligosaccharides Containing Water-Soluble Dietary Fiber
Abstract
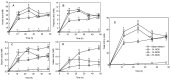
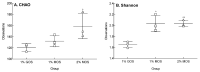
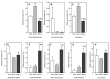
This study measured the proliferative activity of malto-oligosaccharide (MOS) as a prebiotic against Bifidobacteria, resistance to digestion in vitro, and changes during in vitro fermentation by human fecal microorganisms. It consisted of 21.74%, 18.84%, and 11.76% of maltotriose, maltotetraose, and maltopentaose produced by amylase (HATT), respectively. When 1% of MOS was added to a modified PYF medium as the carbon source, proliferation of Bifidobacterium breve was increased significantly. During the in vitro digestion test, MOS was partially degraded by intestinal enzymes. Fermentation characteristics by human fecal microorganisms were evaluated by adding 1% galacto-oligosaccharide (GOS), as well as 1% and 2% MOS as carbon sources to the basal medium, respectively. In comparison with the addition of 1% of MOS and GOS, the total short chain fatty acid (SCFA) content increased over time when 2% of MOS was added. The species diversity and richness of intestinal microbiota increased significantly with 2% MOS compared to those with 1% GOS. In addition, the 2% addition of MOS reduced intestinal pathobiont microorganisms and increased commensal microorganisms including Bifidobacterium genus. Collectively, MOS produced by amylase increased the SCFA production and enhanced the growth of beneficial bacteria during in vitro fermentation by human fecal microbiota.
Keywords: SCFA; in vitro fermentation; malto-oligosaccharide; prebiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Fermentation properties and potential prebiotic activity of Bimuno® galacto-oligosaccharide (65 % galacto-oligosaccharide content) on in vitro gut microbiota parameters.Br J Nutr. 2016 Aug;116(3):480-6. doi: 10.1017/S0007114516002269. Epub 2016 Jun 8. Br J Nutr. 2016. PMID: 27267934 Free PMC article.
-
Generation of novel prebiotic oligosaccharide pools from fiber drives biological insight in bacterial glycan metabolism.Appl Environ Microbiol. 2025 Mar 19;91(3):e0207724. doi: 10.1128/aem.02077-24. Epub 2025 Feb 6. Appl Environ Microbiol. 2025. PMID: 39912642 Free PMC article.
-
Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice.Br J Nutr. 2016 Jul;116(2):270-8. doi: 10.1017/S0007114516001987. Epub 2016 May 20. Br J Nutr. 2016. PMID: 27198516
-
Relationship of prebiotics and food to intestinal microflora.Eur J Nutr. 2002 Nov;41 Suppl 1:I11-6. doi: 10.1007/s00394-002-1102-7. Eur J Nutr. 2002. PMID: 12420111 Review.
-
Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors-A review.Carbohydr Polym. 2022 May 15;284:119043. doi: 10.1016/j.carbpol.2021.119043. Epub 2021 Dec 30. Carbohydr Polym. 2022. PMID: 35287885 Review.
Cited by
-
Molecular Diversity and Biochemical Content in Two Invasive Alien Species: Looking for Chemical Similarities and Bioactivities.Mar Drugs. 2022 Dec 22;21(1):5. doi: 10.3390/md21010005. Mar Drugs. 2022. PMID: 36662178 Free PMC article.
-
Maltooligosaccharides: Properties, Production and Applications.Molecules. 2023 Apr 6;28(7):3281. doi: 10.3390/molecules28073281. Molecules. 2023. PMID: 37050044 Free PMC article. Review.
-
Maltooligosaccharide forming amylases and their applications in food and pharma industry.J Food Sci Technol. 2022 Oct;59(10):3733-3744. doi: 10.1007/s13197-021-05262-7. Epub 2021 Sep 27. J Food Sci Technol. 2022. PMID: 36193376 Free PMC article. Review.
-
Effective Regulation of Gut Microbiota With Probiotics and Prebiotics May Prevent or Alleviate COVID-19 Through the Gut-Lung Axis.Front Pharmacol. 2022 Apr 25;13:895193. doi: 10.3389/fphar.2022.895193. eCollection 2022. Front Pharmacol. 2022. PMID: 35548347 Free PMC article. Review.
-
Targeting the gut microbiota: a new strategy for colorectal cancer treatment.J Transl Med. 2024 Oct 8;22(1):915. doi: 10.1186/s12967-024-05671-0. J Transl Med. 2024. PMID: 39379983 Free PMC article. Review.
References
-
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. - DOI - PubMed
-
- de Paulo Farias D., de Araújo F.F., Neri-Numa I.A., Pastore G.M. Prebiotics: Trends in food, health and technological applications. Trends.Food. Sci. Technol. 2019;93:23–35. doi: 10.1016/j.tifs.2019.09.004. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

