Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation
- PMID: 33182252
- PMCID: PMC7695271
- DOI: 10.3390/antiox9111098
Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation
Abstract
Whether flavonoids play significant antioxidant roles in plants challenged by photooxidative stress of different origin has been largely debated over the last few decades. A critical review of the pertinent literature and our experimentation as well, based on a free-of-scale approach, support an important antioxidant function served by flavonoids in plants exposed to a wide range of environmental stressors, the significance of which increases with the severity of stress. On the other side, some questions need conclusive answers when the putative antioxidant functions of plant flavonoids are examined at the level of both the whole-cell and cellular organelles. This partly depends upon a conclusive, robust, and unbiased definition of "a plant antioxidant", which is still missing, and the need of considering the subcellular re-organization that occurs in plant cells in response to severe stress conditions. This likely makes our deterministic-based approach unsuitable to unveil the relevance of flavonoids as antioxidants in extremely complex biological systems, such as a plant cell exposed to an ever-changing stressful environment. This still poses open questions about how to measure the occurred antioxidant action of flavonoids. Our reasoning also evidences the need of contemporarily evaluating the changes in key primary and secondary components of the antioxidant defense network imposed by stress events of increasing severity to properly estimate the relevance of the antioxidant functions of flavonoids in an in planta situation. In turn, this calls for an in-depth analysis of the sub-cellular distribution of primary and secondary antioxidants to solve this still intricate matter.
Keywords: UV-B radiation; antioxidant enzymes; cytoplasm-located flavonoids; early land plants; flavonols; hydrogen peroxide; photoprotection; reactive oxygen species; vacuolar flavonoids.
Conflict of interest statement
The authors declare no conflict of interest.
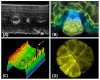
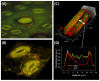
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

