The Arabidopsis L-Type Amino Acid Transporter 5 (LAT5/PUT5) Is Expressed in the Phloem and Alters Seed Nitrogen Content When Knocked Out
- PMID: 33182302
- PMCID: PMC7695346
- DOI: 10.3390/plants9111519
The Arabidopsis L-Type Amino Acid Transporter 5 (LAT5/PUT5) Is Expressed in the Phloem and Alters Seed Nitrogen Content When Knocked Out
Abstract
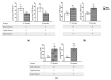
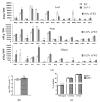
The Arabidopsis L-type Amino Acid Transporter-5 (LAT5; At3g19553) was recently studied for its role in developmental responses such as flowering and senescence, under an assumption that it is a polyamine uptake transporter (PUT5). The LATs in Arabidopsis have a wide range of substrates, including amino acids and polyamines. This report extensively studied the organ and tissue-specific expression of the LAT5/PUT5 and investigated its role in mediating amino acid transport. Organ-specific quantitative RT-PCR detected LAT5/PUT5 transcripts in all organs with a relatively higher abundance in the leaves. Tissue-specific expression analysis identified GUS activity in the phloem under the LAT5/PUT5 promoter. In silico analysis identified both amino acid transporter and antiporter domains conserved in the LAT5/PUT5 protein. The physiological role of the LAT5/PUT5 was studied through analyzing a mutant line, lat5-1, under various growth conditions. The mutant lat5-1 seedlings showed increased sensitivity to exogenous leucine in Murashige and Skoog growth medium. In soil, the lat5-1 showed reduced leaf growth and altered nitrogen content in the seeds. In planta radio-labelled leucine uptake studies showed increased accumulation of leucine in the lat5-1 plants compared to the wild type when treated in the dark prior to the isotopic feeding. These studies suggest that LAT5/PUT5 plays a role in mediating amino acid transport.
Keywords: Arabidopsis; L-type amino acid transporter; LAT; LAT5; PUT5; amino acid translocation; amino acids; nitrogen; polyamine uptake transporter; seed nitrogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Begam R.A. Functional Characterization of the L-Type Amino Acid Transporters (LATs) in Arabidopsis Thaliana. Primary Research, University of Alberta; Edmonton, AB, Canada: 2012.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

