Changes in Striatal Medium Spiny Neuron Morphology Resulting from Dopamine Depletion Are Reversible
- PMID: 33182316
- PMCID: PMC7695336
- DOI: 10.3390/cells9112441
Changes in Striatal Medium Spiny Neuron Morphology Resulting from Dopamine Depletion Are Reversible
Abstract
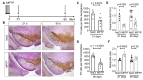
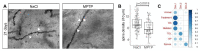
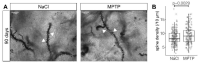
The classical motor symptoms of Parkinson's disease (PD) are caused by degeneration of dopaminergic neurons in the substantia nigra, which is followed by secondary dendritic pruning and spine loss at striatal medium spiny neurons (MSN). We hypothesize that these morphological changes at MSN underlie at least in part long-term motor complications in PD patients. In order to define the potential benefits and limitations of dopamine substitution, we tested in a mouse model whether dendritic pruning and spine loss can be reversible when dopaminergic axon terminals regenerate. In order to induce degeneration of nigrostriatal dopaminergic neurons we used the toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in C57BL/6J mice; 30 mg/kg MPTP was applied i.p. on five consecutive days. In order to assess the consequences of dopamine depletion, mice were analyzed 21 days after the last injection. In order to test reversibility of MSN changes we exploited the property of this model that striatal axon terminals regenerate by sprouting within 90 days and analyzed a second cohort 90 days after MPTP. Degeneration of dopaminergic neurons was confirmed by counting TH-positive neurons in the substantia nigra and by analyzing striatal catecholamines. Striatal catecholamine recovered 90 days after MPTP. MSN morphology was visualized by Golgi staining and quantified as total dendritic length, number of dendritic branch points, and density of dendritic spines. All morphological parameters of striatal MSN were reduced 21 days after MPTP. Statistical analysis indicated that dendritic pruning and the reduction of spine density represent two distinct responses to dopamine depletion. Ninety days after MPTP, all morphological changes recovered. Our findings demonstrate that morphological changes in striatal MSN resulting from dopamine depletion are reversible. They suggest that under optimal conditions, symptomatic dopaminergic therapy might be able to prevent maladaptive plasticity and long-term motor complications in PD patients.
Keywords: dendrite morphology; spine density; spiny projection neurons; striatum.
Conflict of interest statement
The authors declare no conflict of interest
Figures


Similar articles
-
Acute MPTP treatment decreases dendritic spine density of striatal medium spiny neurons via SNK-SPAR pathway in C57BL/6 mice.Synapse. 2022 Sep;76(11-12):e22249. doi: 10.1002/syn.22249. Epub 2022 Aug 30. Synapse. 2022. PMID: 36008099
-
Remodeling of the dendritic structure of the striatal medium spiny neurons accompanies behavioral recovery in a mouse model of Parkinson's disease.Neurosci Lett. 2013 Dec 17;557 Pt B:95-100. doi: 10.1016/j.neulet.2013.10.049. Epub 2013 Oct 28. Neurosci Lett. 2013. PMID: 24176882
-
Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease.Neurobiol Dis. 2014 Mar;63:201-9. doi: 10.1016/j.nbd.2013.11.017. Epub 2013 Dec 5. Neurobiol Dis. 2014. PMID: 24316165 Free PMC article.
-
Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism.Parkinsonism Relat Disord. 2007;13 Suppl 3(Suppl 3):S251-8. doi: 10.1016/S1353-8020(08)70012-9. Parkinsonism Relat Disord. 2007. PMID: 18267246 Free PMC article. Review.
-
Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
Cited by
-
Exercise training upregulates CD55 to suppress complement-mediated synaptic phagocytosis in Parkinson's disease.J Neuroinflammation. 2024 Sep 28;21(1):246. doi: 10.1186/s12974-024-03234-0. J Neuroinflammation. 2024. PMID: 39342308 Free PMC article.
-
Gray matter structural and functional brain abnormalities in Parkinson's disease: a meta-analysis of VBM and ALFF data.J Neurol. 2025 Mar 19;272(4):276. doi: 10.1007/s00415-025-12934-3. J Neurol. 2025. PMID: 40106017 Review.
-
Heterogeneous brain region-specific responses to astrocytic mitochondrial DNA damage in mice.Sci Rep. 2024 Aug 10;14(1):18586. doi: 10.1038/s41598-024-69499-w. Sci Rep. 2024. PMID: 39127716 Free PMC article.
-
An accelerated Parkinson's disease monkey model using AAV-α-synuclein plus poly(ADP-ribose).Cell Rep Methods. 2024 Oct 21;4(10):100876. doi: 10.1016/j.crmeth.2024.100876. Epub 2024 Oct 15. Cell Rep Methods. 2024. PMID: 39413778 Free PMC article.
-
Role of Stress-Related Dopamine Transmission in Building and Maintaining a Protective Cognitive Reserve.Brain Sci. 2022 Feb 11;12(2):246. doi: 10.3390/brainsci12020246. Brain Sci. 2022. PMID: 35204009 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

