Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice
- PMID: 33182364
- PMCID: PMC7695289
- DOI: 10.3390/nu12113432
Kefir Peptides Prevent Estrogen Deficiency-Induced Bone Loss and Modulate the Structure of the Gut Microbiota in Ovariectomized Mice
Abstract
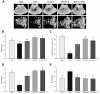
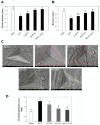
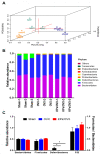
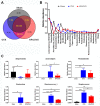
Osteoporosis is a major skeletal disease associated with estrogen deficiency in postmenopausal women. Kefir-fermented peptides (KPs) are bioactive peptides with health-promoting benefits that are produced from the degradation of dairy milk proteins by the probiotic microflora in kefir grains. This study aimed to evaluate the effects of KPs on osteoporosis prevention and the modulation of the composition of the gut microbiota in ovariectomized (OVX) mice. OVX mice receiving an 8-week oral gavage of 100 mg of KPs and 100 mg of KPs + 10 mg Ca exhibited lower trabecular separation (Tb. Sp), and higher bone mineral density (BMD), trabecular number (Tb. N) and bone volume (BV/TV), than OVX groups receiving Ca alone and untreated mice, and these effects were also reflected in bones with better mechanical properties of strength and fracture toughness. The gut microbiota of the cecal contents was examined by 16S rDNA amplicon sequencing. α-Diversity analysis indicated that the gut microbiota of OVX mice was enriched more than that of sham mice, but the diversity was not changed significantly. Treatment with KPs caused increased microbiota richness and diversity in OVX mice compared with those in sham mice. The microbiota composition changed markedly in OVX mice compared with that in sham mice. Following the oral administration of KPs for 8 weeks, the abundances of Alloprevotella, Anaerostipes, Parasutterella, Romboutsia, Ruminococcus_1 and Streptococcus genera were restored to levels close to those in the sham group. However, the correlation of these bacterial populations with bone metabolism needs further investigation. Taken together, KPs prevent menopausal osteoporosis and mildly modulate the structure of the gut microbiota in OVX mice.
Keywords: 16S rDNA; dairy milk protein; gut microbiota; kefir peptides; osteoporosis; ovariectomized (OVX) mice.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

