Antimicrobial Peptide against Mycobacterium Tuberculosis That Activates Autophagy Is an Effective Treatment for Tuberculosis
- PMID: 33182483
- PMCID: PMC7697726
- DOI: 10.3390/pharmaceutics12111071
Antimicrobial Peptide against Mycobacterium Tuberculosis That Activates Autophagy Is an Effective Treatment for Tuberculosis
Abstract
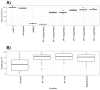
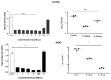
Mycobacterium tuberculosis (MTB) is the principal cause of human tuberculosis (TB), which is a serious health problem worldwide. The development of innovative therapeutic modalities to treat TB is mainly due to the emergence of multi drug resistant (MDR) TB. Autophagy is a cell-host defense process. Previous studies have reported that autophagy-activating agents eliminate intracellular MDR MTB. Thus, combining a direct antibiotic activity against circulating bacteria with autophagy activation to eliminate bacteria residing inside cells could treat MDR TB. We show that the synthetic peptide, IP-1 (KFLNRFWHWLQLKPGQPMY), induced autophagy in HEK293T cells and macrophages at a low dose (10 μM), while increasing the dose (50 μM) induced cell death; IP-1 induced the secretion of TNFα in macrophages and killed Mtb at a dose where macrophages are not killed by IP-1. Moreover, IP-1 showed significant therapeutic activity in a mice model of progressive pulmonary TB. In terms of the mechanism of action, IP-1 sequesters ATP in vitro and inside living cells. Thus, IP-1 is the first antimicrobial peptide that eliminates MDR MTB infection by combining four activities: reducing ATP levels, bactericidal activity, autophagy activation, and TNFα secretion.
Keywords: antimicrobial peptide; autophagy; iztli peptide; multidrug resistant; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Barbier M., Wirth T. Tuberculosis and the Tubercle Bacillus. 2nd ed. American Society of Microbiology; Washington, DC, USA: 2016. The Evolutionary History, Demography, and Spread of the Mycobacterium tuberculosis Complex; pp. 453–473. - PubMed
Grants and funding
- IG100513/Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México
- IG100416/Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México
- IN208014/Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México
- IN208817/Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México
- IN206518/Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

