Novel FGFR4-Targeting Single-Domain Antibodies for Multiple Targeted Therapies against Rhabdomyosarcoma
- PMID: 33182650
- PMCID: PMC7696840
- DOI: 10.3390/cancers12113313
Novel FGFR4-Targeting Single-Domain Antibodies for Multiple Targeted Therapies against Rhabdomyosarcoma
Abstract
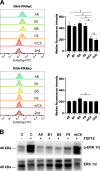
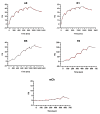
The fibroblast growth factor receptor 4 (FGFR4) is overexpressed in rhabdomyosarcoma (RMS) and represents a promising target for treatments based on specific and efficient antibodies. Despite progress, there is an urgent need for targeted treatment options to improve survival rates, and to limit long-term side effects. From phage display libraries we selected FGFR4-specific single-domain antibodies (sdAb) binding to recombinant FGFR4 and validated them by flow cytometry, surface plasmon resonance, and fluorescence microscopy. The specificity of the selected sdAb was verified on FGFR4-wild type and FGFR4-knock out cells. FGFR4-sdAb were used to decorate vincristine-loaded liposomes and to generate chimeric antigen receptor (CAR) T cells. First, incubation of RMS cells with FGFR4-sdAb revealed that FGFR4-sdAb can block FGF19-FGFR4 signaling via the MAPK pathway and could therefore serve as therapeutics for FGFR4-dependent cancers. Second, FGFR4-targeted vincristine-loaded liposomes bound specifically to RMS cells and were internalized by the receptor, demonstrating the potential for active drug delivery to the tumor. Third, FGFR4-CAR T cells, generated with one sdAb candidate, demonstrated strong and specific cytotoxicity against FGFR4 expressing RMS cells. We selected novel FGFR4-sdAb with high specificity and nano- to picomolar affinities for FGFR4 which have the potential to enable multiple FGFR4-targeted cancer therapy approaches.
Keywords: CAR T cells; FGFR4; rhabdomyosarcoma; single-domain antibody; targeted liposomes.
Conflict of interest statement
N.A., S.M., Z.G., F.P. and M.B. have filed a patent to protect the commercial use of these data. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
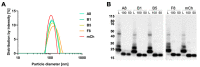

References
-
- Hern J.F., Chen L., Chmielecki J., Wei J.S., Patidar R., Rosenberg M., Ambrogio L., Auclair D., Wang J., Song Y.K., et al. Comprehensive Genomic Analysis of Rhabdomyosarcoma Reveals a Landscape of Alterations Affecting a Common Genetic Axis in Fusion-Positive and Fusion-Negative Tumors. Cancer Discov. 2014;4:216–231. doi: 10.1158/2159-8290.cd-13-0639. - DOI - PMC - PubMed
-
- Van Gaal J.C., Van Der Graaf W.T.A., Rikhof B., Van Hoesel Q.G.C.M., Teerenstra S., Suurmeijer A.J.H., Flucke U.E., Loeffen J.L.C.M., Sleijfer S., De Bont E.S.J.M. The impact of age on outcome of embryonal and alveolar rhabdomyosarcoma patients. A multicenter study. Anticancer Res. 2012;32:4485–4497. - PubMed
-
- Punyko J.A., Mertens A.C., Gurney J.G., Yasui Y., Donaldson S.S., Rodeberg D.A., Raney R.B., Stovall M., Sklar C.A., Robison L.L., et al. Long-term medical effects of childhood and adolescent rhabdomyosarcoma: A report from the childhood cancer survivor study. Pediatr. Blood Cancer. 2005;44:643–653. doi: 10.1002/pbc.20310. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

