Animal Models: A Useful Tool to Unveil Metabolic Changes in Hepatocellular Carcinoma
- PMID: 33182674
- PMCID: PMC7696782
- DOI: 10.3390/cancers12113318
Animal Models: A Useful Tool to Unveil Metabolic Changes in Hepatocellular Carcinoma
Abstract
Hepatocellular carcinoma (HCC) is one the most frequent and lethal human cancers. At present, no effective treatment for advanced HCC exist; therefore, the overall prognosis for HCC patients remains dismal. In recent years, a better knowledge of the signaling pathways involved in the regulation of HCC development and progression, has led to the identification of novel potential targets for therapeutic strategies. However, the obtained benefits from current therapeutic options are disappointing. Altered cancer metabolism has become a topic of renewed interest in the last decades, and it has been included among the core hallmarks of cancer. In the light of growing evidence for metabolic reprogramming in cancer, a wide number of experimental animal models have been exploited to study metabolic changes characterizing HCC development and progression and to further expand our knowledge of this tumor. In the present review, we discuss several rodent models of hepatocarcinogenesis, that contributed to elucidate the metabolic profile of HCC and the implications of these changes in modulating the aggressiveness of neoplastic cells. We also highlight the apparently contrasting results stemming from different animal models. Finally, we analyze whether these observations could be exploited to improve current therapeutic strategies for HCC.
Keywords: HCC; OXPHOS; PPP; glycolysis; miRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
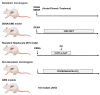

 Hydrodynamic Injection.
Hydrodynamic Injection.  Cancer cells. The figure has been prepared by adapting BioRender images.
Cancer cells. The figure has been prepared by adapting BioRender images.
References
-
- Finn R.S., Merle P., Granito A., Huang Y.H., Bodoky G., Pracht M., Yokosuka O., Rosmorduc O., Gerolami R., Caparello C., et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J. Hepatol. 2018;69:353–358. doi: 10.1016/j.jhep.2018.04.010. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

