Corneal Infection Models: Tools to Investigate the Role of Biofilms in Bacterial Keratitis
- PMID: 33182687
- PMCID: PMC7696224
- DOI: 10.3390/cells9112450
Corneal Infection Models: Tools to Investigate the Role of Biofilms in Bacterial Keratitis
Abstract
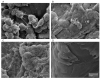
Bacterial keratitis is a corneal infection which may cause visual impairment or even loss of the infected eye. It remains a major cause of blindness in the developing world. Staphylococcus aureus and Pseudomonas aeruginosa are common causative agents and these bacterial species are known to colonise the corneal surface as biofilm populations. Biofilms are complex bacterial communities encased in an extracellular polymeric matrix and are notoriously difficult to eradicate once established. Biofilm bacteria exhibit different phenotypic characteristics from their planktonic counterparts, including an increased resistance to antibiotics and the host immune response. Therefore, understanding the role of biofilms will be essential in the development of new ophthalmic antimicrobials. A brief overview of biofilm-specific resistance mechanisms is provided, but this is a highly multifactorial and rapidly expanding field that warrants further research. Progression in this field is dependent on the development of suitable biofilm models that acknowledge the complexity of the ocular environment. Abiotic models of biofilm formation (where biofilms are studied on non-living surfaces) currently dominate the literature, but co-culture infection models are beginning to emerge. In vitro, ex vivo and in vivo corneal infection models have now been reported which use a variety of different experimental techniques and animal models. In this review, we will discuss existing corneal infection models and their application in the study of biofilms and host-pathogen interactions at the corneal surface.
Keywords: bacterial keratitis; biofilm; cornea; ex vivo; in vitro; in vivo; infection; microbial keratitis; models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

