Photochemotherapy Induces Interferon Type III Expression via STING Pathway
- PMID: 33182724
- PMCID: PMC7697763
- DOI: 10.3390/cells9112452
Photochemotherapy Induces Interferon Type III Expression via STING Pathway
Abstract
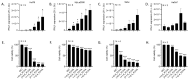
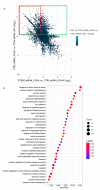
DNA-damaging cancer therapies induce interferon expression and stimulate the immune system, promoting therapy responses. The immune-activating STING (Stimulator of Interferon Genes) pathway is induced when DNA or double-stranded RNA (dsRNA) is detected in the cell cytoplasm, which can be caused by viral infection or by DNA damage following chemo- or radiotherapy. Here, we investigated the responses of cutaneous T-cell lymphoma (CTCL) cells to the clinically applied DNA crosslinking photochemotherapy (combination of 8-methoxypsoralen and UVA light; 8-MOP + UVA). We showed that this treatment evokes interferon expression and that the type III interferon IFNL1 is the major cytokine induced. IFNL1 upregulation is dependent on STING and on the cytoplasmic DNA sensor cyclic GMP-AMP synthase (cGAS). Furthermore, 8-MOP + UVA treatment induced the expression of genes in pathways involved in response to the tumor necrosis factor, innate immune system and acute inflammatory response. Notably, a subset of these genes was under control of the STING-IFNL1 pathway. In conclusion, our data connected DNA damage with immune system activation via the STING pathway and contributed to a better understanding of the effectiveness of photochemotherapy.
Keywords: CTCL; DNA damage; IFNL1; PUVA; STING.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

