Mitochondrial Miro GTPases coordinate mitochondrial and peroxisomal dynamics
- PMID: 33183150
- PMCID: PMC8583064
- DOI: 10.1080/21541248.2020.1843957
Mitochondrial Miro GTPases coordinate mitochondrial and peroxisomal dynamics
Abstract
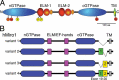
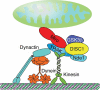
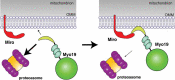
Mitochondria and peroxisomes are highly dynamic, multifunctional organelles. Both perform key roles for cellular physiology and homoeostasis by mediating bioenergetics, biosynthesis, and/or signalling. To support cellular function, they must be properly distributed, of proper size, and be able to interact with other organelles. Accumulating evidence suggests that the small atypical GTPase Miro provides a central signalling node to coordinate mitochondrial as well as peroxisomal dynamics. In this review, I summarize our current understanding of Miro-dependent functions and molecular mechanisms underlying the proper distribution, size and function of mitochondria and peroxisomes.
Keywords: Miro; Small GTPase; membrane dynamics; mitochondria; mitochondrial dynamics; motility; peroxisomal dynamics; peroxisome.
Conflict of interest statement
I have no potential conflicts of interest.
Figures

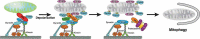

Similar articles
-
The Miro GTPases: at the heart of the mitochondrial transport machinery.FEBS Lett. 2009 May 6;583(9):1391-8. doi: 10.1016/j.febslet.2009.04.015. Epub 2009 Apr 18. FEBS Lett. 2009. PMID: 19376118 Review.
-
Miro GTPases at the Crossroads of Cytoskeletal Dynamics and Mitochondrial Trafficking.Cells. 2024 Apr 7;13(7):647. doi: 10.3390/cells13070647. Cells. 2024. PMID: 38607086 Free PMC article. Review.
-
Fission and proliferation of peroxisomes.Biochim Biophys Acta. 2012 Sep;1822(9):1343-57. doi: 10.1016/j.bbadis.2011.12.014. Epub 2011 Dec 31. Biochim Biophys Acta. 2012. PMID: 22240198 Review.
-
VPS13D bridges the ER to mitochondria and peroxisomes via Miro.J Cell Biol. 2021 May 3;220(5):e202010004. doi: 10.1083/jcb.202010004. J Cell Biol. 2021. PMID: 33891013 Free PMC article.
-
Fission Impossible (?)-New Insights into Disorders of Peroxisome Dynamics.Cells. 2022 Jun 14;11(12):1922. doi: 10.3390/cells11121922. Cells. 2022. PMID: 35741050 Free PMC article. Review.
Cited by
-
Highly Specialized Mechanisms for Mitochondrial Transport in Neurons: From Intracellular Mobility to Intercellular Transfer of Mitochondria.Biomolecules. 2023 Jun 3;13(6):938. doi: 10.3390/biom13060938. Biomolecules. 2023. PMID: 37371518 Free PMC article. Review.
-
Mitochondrial quality control in diabetic cardiomyopathy: from molecular mechanisms to therapeutic strategies.Int J Biol Sci. 2022 Aug 15;18(14):5276-5290. doi: 10.7150/ijbs.75402. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147470 Free PMC article. Review.
-
Systematic investigation of mitochondrial transfer between cancer cells and T cells at single-cell resolution.Cancer Cell. 2023 Oct 9;41(10):1788-1802.e10. doi: 10.1016/j.ccell.2023.09.003. Cancer Cell. 2023. PMID: 37816332 Free PMC article.
-
Miro proteins and their role in mitochondrial transfer in cancer and beyond.Front Cell Dev Biol. 2022 Jul 25;10:937753. doi: 10.3389/fcell.2022.937753. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35959487 Free PMC article. Review.
-
Mitochondrial membrane synthesis, remodelling and cellular trafficking.J Inherit Metab Dis. 2025 Jan;48(1):e12766. doi: 10.1002/jimd.12766. Epub 2024 Jun 14. J Inherit Metab Dis. 2025. PMID: 38872485 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
