Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility
- PMID: 33183171
- PMCID: PMC7856104
- DOI: 10.1161/CIRCRESAHA.120.317452
Intracellular β1-Adrenergic Receptors and Organic Cation Transporter 3 Mediate Phospholamban Phosphorylation to Enhance Cardiac Contractility
Abstract
Rationale: β1ARs (β1-adrenoceptors) exist at intracellular membranes and OCT3 (organic cation transporter 3) mediates norepinephrine entry into cardiomyocytes. However, the functional role of intracellular β1AR in cardiac contractility remains to be elucidated.
Objective: Test localization and function of intracellular β1AR on cardiac contractility.
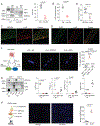
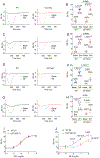
Methods and results: Membrane fractionation, super-resolution imaging, proximity ligation, coimmunoprecipitation, and single-molecule pull-down demonstrated a pool of β1ARs in mouse hearts that were associated with sarco/endoplasmic reticulum Ca2+-ATPase at the sarcoplasmic reticulum (SR). Local PKA (protein kinase A) activation was measured using a PKA biosensor targeted at either the plasma membrane (PM) or SR. Compared with wild-type, myocytes lacking OCT3 (OCT3-KO [OCT3 knockout]) responded identically to the membrane-permeant βAR agonist isoproterenol in PKA activation at both PM and SR. The same was true at the PM for membrane-impermeant norepinephrine, but the SR response to norepinephrine was suppressed in OCT3-KO myocytes. This differential effect was recapitulated in phosphorylation of the SR-pump regulator phospholamban. Similarly, OCT3-KO selectively suppressed calcium transients and contraction responses to norepinephrine but not isoproterenol. Furthermore, sotalol, a membrane-impermeant βAR-blocker, suppressed isoproterenol-induced PKA activation at the PM but permitted PKA activation at the SR, phospholamban phosphorylation, and contractility. Moreover, pretreatment with sotalol in OCT3-KO myocytes prevented norepinephrine-induced PKA activation at both PM and the SR and contractility.
Conclusions: Functional β1ARs exists at the SR and is critical for PKA-mediated phosphorylation of phospholamban and cardiac contractility upon catecholamine stimulation. Activation of these intracellular β1ARs requires catecholamine transport via OCT3.
Keywords: catecholamine; intracellular membrane; norepinephrine; phospholamban; phosphorylation.
Figures





References
-
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG and Hebert TE. Functional beta-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69–78. - PubMed
-
- Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E and Lavandero S. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res. 2013;112:236–45. - PubMed
-
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria-Gomez E, Matias I, Delamarre A, Metna-Laurent M, Cannich A, Hebert-Chatelain E, Mulle C, Ortega-Gutierrez S, Martin-Fontecha M, Klugmann M, Guggenhuber S, Lutz B, Gertsch J, Chaouloff F, Lopez-Rodriguez ML, Grandes P, Rossignol R and Marsicano G. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nature neuroscience. 2012;15:558–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

