Key role for lipids in cognitive symptoms of schizophrenia
- PMID: 33184259
- PMCID: PMC7665187
- DOI: 10.1038/s41398-020-01084-x
Key role for lipids in cognitive symptoms of schizophrenia
Abstract
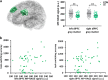
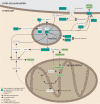
Schizophrenia (SZ) is a psychiatric disorder with a convoluted etiology that includes cognitive symptoms, which arise from among others a dysfunctional dorsolateral prefrontal cortex (dlPFC). In our search for the molecular underpinnings of the cognitive deficits in SZ, we here performed RNA sequencing of gray matter from the dlPFC of SZ patients and controls. We found that the differentially expressed RNAs were enriched for mRNAs involved in the Liver X Receptor/Retinoid X Receptor (LXR/RXR) lipid metabolism pathway. Components of the LXR/RXR pathway were upregulated in gray matter but not in white matter of SZ dlPFC. Intriguingly, an analysis for shared genetic etiology, using two SZ genome-wide association studies (GWASs) and GWAS data for 514 metabolites, revealed genetic overlap between SZ and acylcarnitines, VLDL lipids, and fatty acid metabolites, which are all linked to the LXR/RXR signaling pathway. Furthermore, analysis of structural T1-weighted magnetic resonance imaging in combination with cognitive behavioral data showed that the lipid content of dlPFC gray matter is lower in SZ patients than in controls and correlates with a tendency towards reduced accuracy in the dlPFC-dependent task-switching test. We conclude that aberrations in LXR/RXR-regulated lipid metabolism lead to a decreased lipid content in SZ dlPFC that correlates with reduced cognitive performance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Association of Age at Onset and Longitudinal Course of Prefrontal Function in Youth With Schizophrenia.JAMA Psychiatry. 2018 Dec 1;75(12):1252-1260. doi: 10.1001/jamapsychiatry.2018.2538. JAMA Psychiatry. 2018. PMID: 30285056 Free PMC article.
-
Altered Associations Between Task Performance and Dorsolateral Prefrontal Cortex Activation During Cognitive Control in Schizophrenia.Biol Psychiatry Cogn Neurosci Neuroimaging. 2023 Oct;8(10):1050-1057. doi: 10.1016/j.bpsc.2023.05.010. Epub 2023 Jun 7. Biol Psychiatry Cogn Neurosci Neuroimaging. 2023. PMID: 37295646 Free PMC article.
-
Shared Genetic Risk of Schizophrenia and Gray Matter Reduction in 6p22.1.Schizophr Bull. 2019 Jan 1;45(1):222-232. doi: 10.1093/schbul/sby010. Schizophr Bull. 2019. PMID: 29474680 Free PMC article.
-
Mechanisms underlying dorsolateral prefrontal cortex contributions to cognitive dysfunction in schizophrenia.Neuropsychopharmacology. 2022 Jan;47(1):292-308. doi: 10.1038/s41386-021-01089-0. Epub 2021 Jul 20. Neuropsychopharmacology. 2022. PMID: 34285373 Free PMC article. Review.
-
Recent Reports on Redox Stress-Induced Mitochondrial DNA Variations, Neuroglial Interactions, and NMDA Receptor System in Pathophysiology of Schizophrenia.Mol Neurobiol. 2022 Apr;59(4):2472-2496. doi: 10.1007/s12035-021-02703-4. Epub 2022 Jan 27. Mol Neurobiol. 2022. PMID: 35083660 Review.
Cited by
-
Brain metabolic profiling of schizophrenia: a path towards a better understanding of the neuropathogenesis of psychosis.Metab Brain Dis. 2024 Nov 21;40(1):28. doi: 10.1007/s11011-024-01447-z. Metab Brain Dis. 2024. PMID: 39570439 Review.
-
Schizophrenia is associated with altered DNA methylation variance.Mol Psychiatry. 2025 Apr;30(4):1383-1395. doi: 10.1038/s41380-024-02749-5. Epub 2024 Sep 13. Mol Psychiatry. 2025. PMID: 39271751 Free PMC article.
-
Increased Complement C4 in a Sparse Neuronal Subset Induces Network-Wide Transcriptomic Alterations in the Prefrontal Cortex.bioRxiv [Preprint]. 2025 May 29:2025.05.29.656749. doi: 10.1101/2025.05.29.656749. bioRxiv. 2025. PMID: 40502190 Free PMC article. Preprint.
-
Glucose homeostasis and cognitive functions in schizophrenia: a systematic review and meta-analysis.Sci Rep. 2025 Jul 2;15(1):22898. doi: 10.1038/s41598-025-06225-0. Sci Rep. 2025. PMID: 40594261 Free PMC article.
-
Interaction between the brain and multiple organ systems in schizophrenia.World J Psychiatry. 2025 Aug 19;15(8):107696. doi: 10.5498/wjp.v15.i8.107696. eCollection 2025 Aug 19. World J Psychiatry. 2025. PMID: 40837794 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

