3D-printed cellular tips for tuning fork atomic force microscopy in shear mode
- PMID: 33184281
- PMCID: PMC7661501
- DOI: 10.1038/s41467-020-19536-9
3D-printed cellular tips for tuning fork atomic force microscopy in shear mode
Abstract
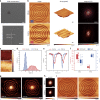
Conventional atomic force microscopy (AFM) tips have remained largely unchanged in nanomachining processes, constituent materials, and microstructural constructions for decades, which limits the measurement performance based on force-sensing feedbacks. In order to save the scanning images from distortions due to excessive mechanical interactions in the intermittent shear-mode contact between scanning tips and sample, we propose the application of controlled microstructural architectured material to construct AFM tips by exploiting material-related energy-absorbing behavior in response to the tip-sample impact, leading to visual promotions of imaging quality. Evidenced by numerical analysis of compressive responses and practical scanning tests on various samples, the essential scanning functionality and the unique contribution of the cellular buffer layer to imaging optimization are strongly proved. This approach opens new avenues towards the specific applications of cellular solids in the energy-absorption field and sheds light on novel AFM studies based on 3D-printed tips possessing exotic properties.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
High-resolution noncontact atomic force microscopy.Nanotechnology. 2009 Jul 1;20(26):260201. doi: 10.1088/0957-4484/20/26/260201. Epub 2009 Jun 10. Nanotechnology. 2009. PMID: 19531843
-
Angled long tip to tuning fork probes for atomic force microscopy in various environments.Rev Sci Instrum. 2011 Apr;82(4):043701. doi: 10.1063/1.3569765. Rev Sci Instrum. 2011. PMID: 21529007
-
Piezoelectric tuning fork probe for atomic force microscopy imaging and specific recognition force spectroscopy of an enzyme and its ligand.J Mol Recognit. 2013 Nov;26(11):521-31. doi: 10.1002/jmr.2294. J Mol Recognit. 2013. PMID: 24089359
-
3D Generation of Multipurpose Atomic Force Microscopy Tips.Adv Sci (Weinh). 2022 Sep;9(27):e2201489. doi: 10.1002/advs.202201489. Epub 2022 Jul 19. Adv Sci (Weinh). 2022. PMID: 35853246 Free PMC article.
-
Chemical modifications of atomic force microscopy tips.Methods Mol Biol. 2011;736:457-83. doi: 10.1007/978-1-61779-105-5_28. Methods Mol Biol. 2011. PMID: 21660744 Review.
Cited by
-
Fully bio-based composite and modular metastructures.Adv Compos Hybrid Mater. 2025;8(4):288. doi: 10.1007/s42114-025-01359-1. Epub 2025 Jul 1. Adv Compos Hybrid Mater. 2025. PMID: 40612640 Free PMC article.
-
Micro 3D printing of a functional MEMS accelerometer.Microsyst Nanoeng. 2022 Sep 19;8:105. doi: 10.1038/s41378-022-00440-9. eCollection 2022. Microsyst Nanoeng. 2022. PMID: 36133693 Free PMC article.
-
Tailored Microcantilever Optimization for Multifrequency Force Microscopy.Adv Sci (Weinh). 2023 Nov;10(33):e2303476. doi: 10.1002/advs.202303476. Epub 2023 Oct 22. Adv Sci (Weinh). 2023. PMID: 37867232 Free PMC article.
-
Measurement of Interfacial Adhesion Force with a 3D-Printed Fiber-Tip Microforce Sensor.Biosensors (Basel). 2022 Aug 11;12(8):629. doi: 10.3390/bios12080629. Biosensors (Basel). 2022. PMID: 36005024 Free PMC article.
-
Fiber-tip polymer clamped-beam probe for high-sensitivity nanoforce measurements.Light Sci Appl. 2021 Aug 27;10(1):171. doi: 10.1038/s41377-021-00611-9. Light Sci Appl. 2021. PMID: 34453031 Free PMC article.
References
-
- Knoll A, Magerle R, Krausch G. Tapping mode atomic force microscopy on polymers: where is the true sample surface. Macromolecules. 2001;34:4159–4165. doi: 10.1021/ma001311x. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

