Molecular structure and interactions within amyloid-like fibrils formed by a low-complexity protein sequence from FUS
- PMID: 33184287
- PMCID: PMC7665218
- DOI: 10.1038/s41467-020-19512-3
Molecular structure and interactions within amyloid-like fibrils formed by a low-complexity protein sequence from FUS
Abstract
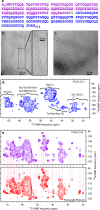
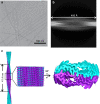
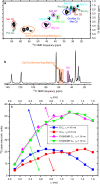
Protein domains without the usual distribution of amino acids, called low complexity (LC) domains, can be prone to self-assembly into amyloid-like fibrils. Self-assembly of LC domains that are nearly devoid of hydrophobic residues, such as the 214-residue LC domain of the RNA-binding protein FUS, is particularly intriguing from the biophysical perspective and is biomedically relevant due to its occurrence within neurons in amyotrophic lateral sclerosis, frontotemporal dementia, and other neurodegenerative diseases. We report a high-resolution molecular structural model for fibrils formed by the C-terminal half of the FUS LC domain (FUS-LC-C, residues 111-214), based on a density map with 2.62 Å resolution from cryo-electron microscopy (cryo-EM). In the FUS-LC-C fibril core, residues 112-150 adopt U-shaped conformations and form two subunits with in-register, parallel cross-β structures, arranged with quasi-21 symmetry. All-atom molecular dynamics simulations indicate that the FUS-LC-C fibril core is stabilized by a plethora of hydrogen bonds involving sidechains of Gln, Asn, Ser, and Tyr residues, both along and transverse to the fibril growth direction, including diverse sidechain-to-backbone, sidechain-to-sidechain, and sidechain-to-water interactions. Nuclear magnetic resonance measurements additionally show that portions of disordered residues 151-214 remain highly dynamic in FUS-LC-C fibrils and that fibrils formed by the N-terminal half of the FUS LC domain (FUS-LC-N, residues 2-108) have the same core structure as fibrils formed by the full-length LC domain. These results contribute to our understanding of the molecular structural basis for amyloid formation by FUS and by LC domains in general.
Conflict of interest statement
The authors have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

