Laser scanning reflection-matrix microscopy for aberration-free imaging through intact mouse skull
- PMID: 33184297
- PMCID: PMC7665219
- DOI: 10.1038/s41467-020-19550-x
Laser scanning reflection-matrix microscopy for aberration-free imaging through intact mouse skull
Abstract
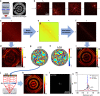
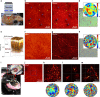
A mouse skull is a barrier for high-resolution optical imaging because its thick and inhomogeneous internal structures induce complex aberrations varying drastically from position to position. Invasive procedures creating either thinned-skull or open-skull windows are often required for the microscopic imaging of brain tissues underneath. Here, we propose a label-free imaging modality termed laser scanning reflection-matrix microscopy for recording the amplitude and phase maps of reflected waves at non-confocal points as well as confocal points. The proposed method enables us to find and computationally correct up to 10,000 angular modes of aberrations varying at every 10 × 10 µm2 patch in the sample plane. We realized reflectance imaging of myelinated axons in vivo underneath an intact mouse skull, with an ideal diffraction-limited spatial resolution of 450 nm. Furthermore, we demonstrated through-skull two-photon fluorescence imaging of neuronal dendrites and their spines by physically correcting the aberrations identified from the reflection matrix.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Minsky, M. Microscopy apparatus. US Patent 3013467 Vol. 3013467 5 (1957).
-
- Dunsby C, French PMW. Techniques for depth-resolved imaging through turbid media including coherence-gated imaging. J. Phys. D. Appl. Phys. 2003;36:R207–R227. doi: 10.1088/0022-3727/36/14/201. - DOI
-
- Pawley, J. Handbook of Biological Confocal Microscopy (Springer, US, 2010).
-
- Booth MJ. Adaptive optical microscopy: the ongoing quest for a perfect image. Light Sci. Amp; Appl. 2014;3:e165. doi: 10.1038/lsa.2014.46. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

